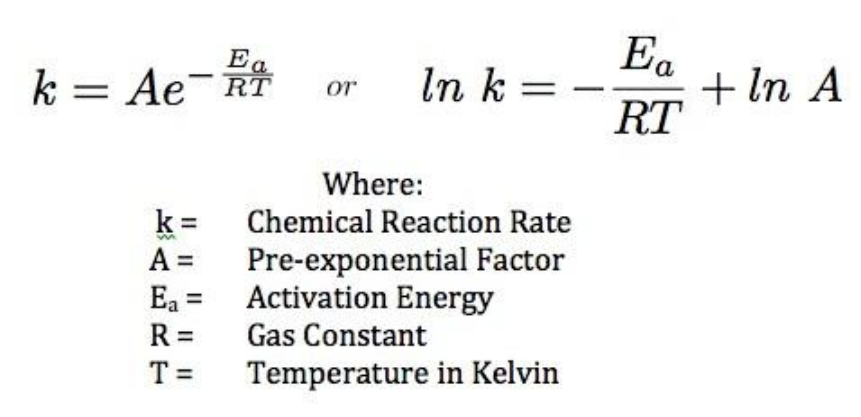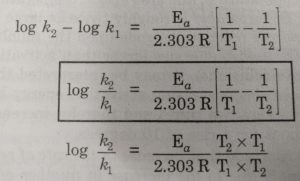Favorite Activation Energy Formula

The final formula to be used can be derived as.
Activation energy formula. There is some formation of bonds or breaking of bond in the chemical reaction which also require some minimum amount of energy. Derivation of the Activation Energy Formula The activation energy formula certainly is k Consider a situation where one rearranges this formula and takes the natural log of this particular. T T is the absolute temperature in Kelvin.
It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation. The plot will form a straight line expressed by the equation. E a the activation energy of the reaction in Jmol R the ideal gas constant 83145 JKmol T 1 and T 2 absolute temperatures Kelvin.
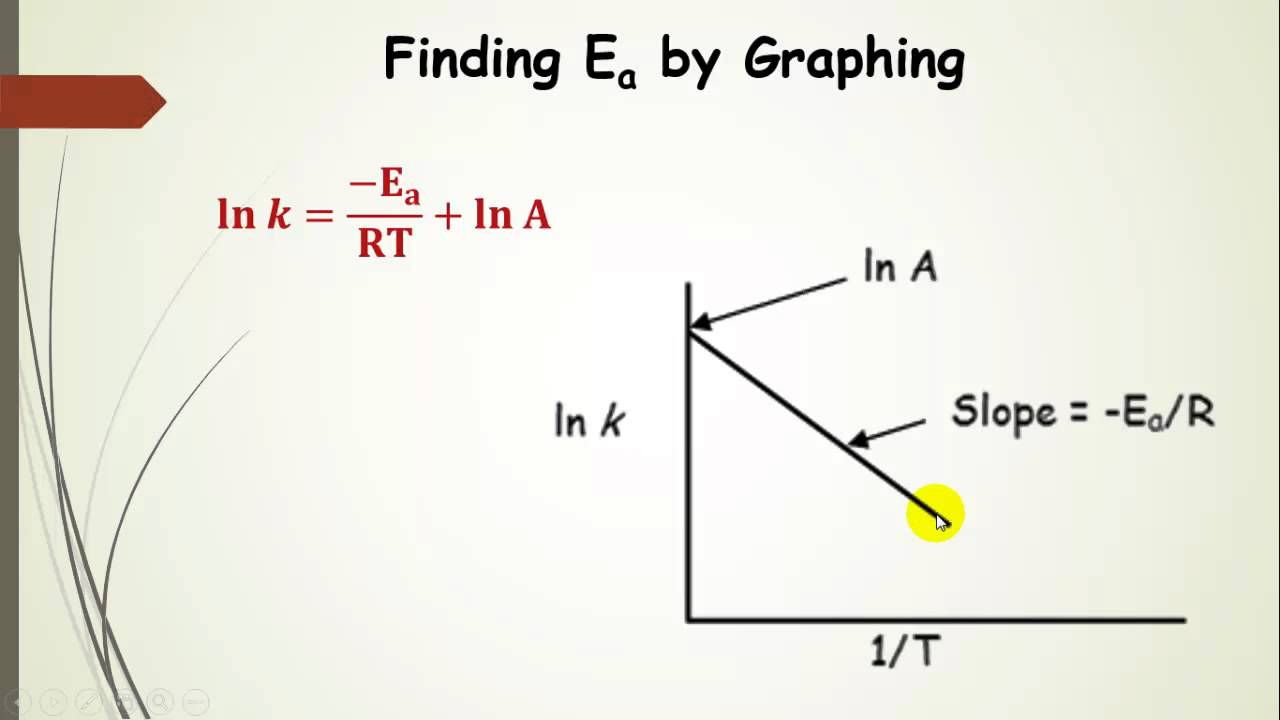
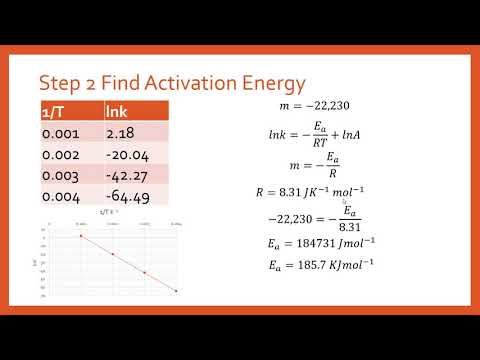
Another way to calculate the activation energy of a reaction is to graph ln k the rate constant versus 1T the inverse of the temperature in Kelvin. What I do not understand is how I can figure this when the given formula for find Ea involves 2 unknown rate constants and the formula for finding the rate constant given involves concentrations. Y is lnk x is 1T and m is -EaR.
R R is the universal gas constant. The activation energy Ea of a reaction is measured in joules J kilojoules per mole kJmol or kilocalories per mole kcalmol. Activation energy is defined as the minimum amount of extra energy required by a reacting molecule to get converted into product.
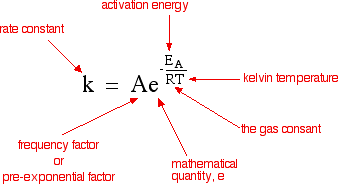
62339 k A e E a R T. A plot of the natural logarithm of k versus 1 T is a straight line with a slope of Ea R. K A e E a R T ln k E a R T ln A.
K Ae Ea RT. The fraction of molecules with energy equal to or greater than E a is given by the exponential term e E a R T in the Arrhenius equation. The activation energy for the reaction can be determined by finding the slope of the line.