Supreme Is Rust A Synthesis Reaction

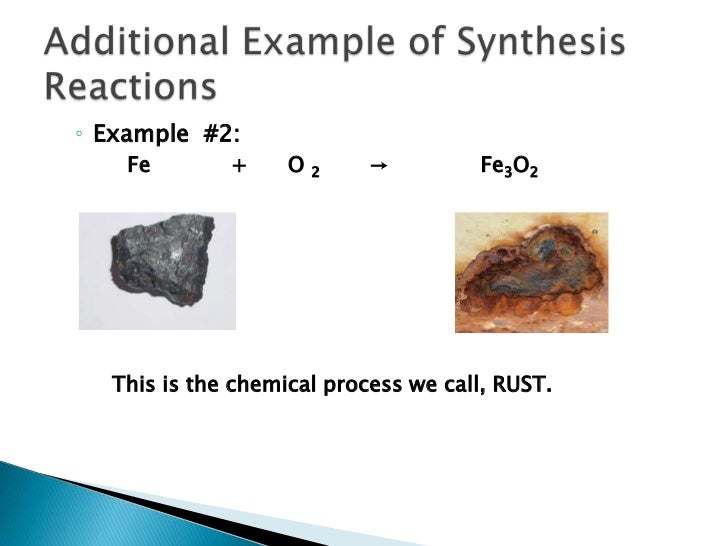
The formation of rust requires iron water and oxygen.
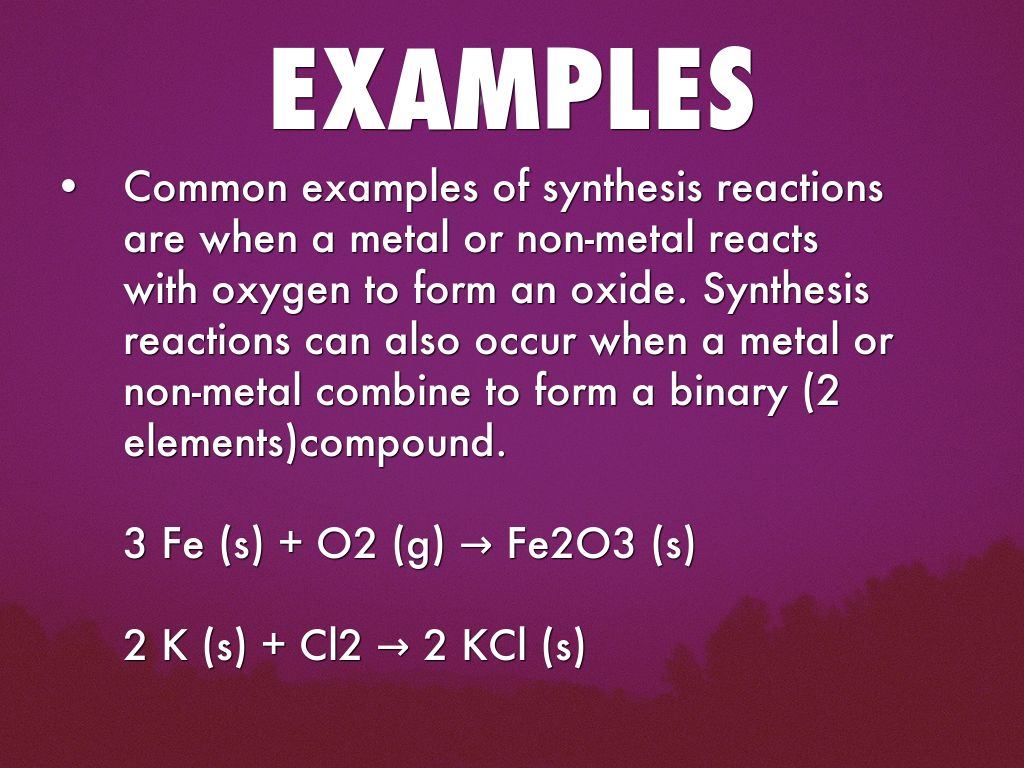
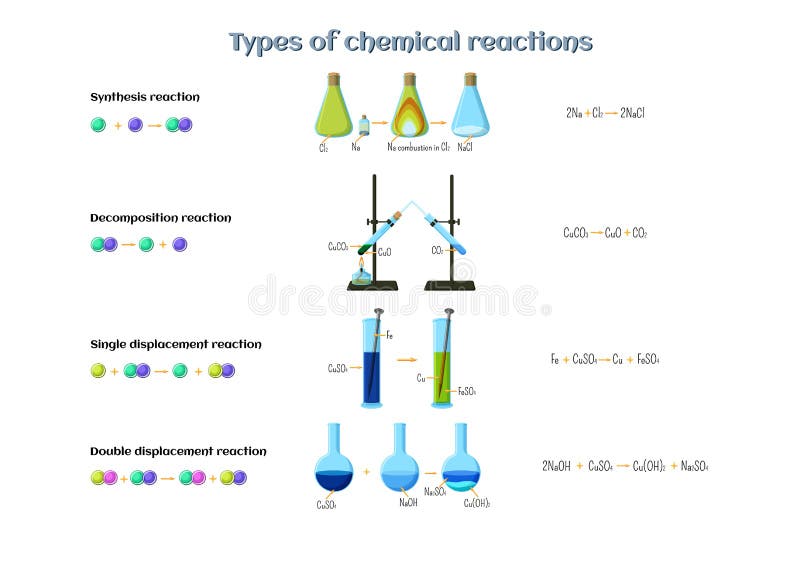
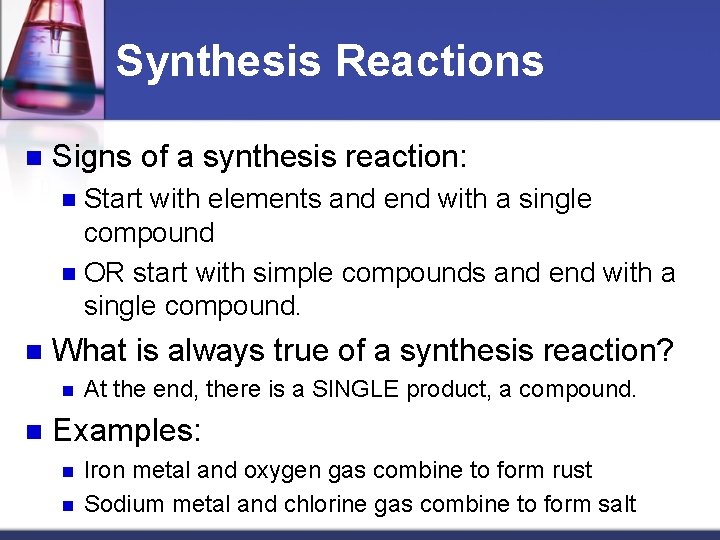
Is rust a synthesis reaction. The overall chemical equation for the formation of rust is. A synthesis reaction occurs when two or more reactants combine to form a single product. Most of the time when a synthesis reaction occurs energy is released and the reaction is exothermic.
Iron Oxygen from environment Water Humidity Iron Oxide Rust. The second step in the corrosion process is also a synthesis reaction. The carbon in steel is added to toughen the steel and wont prevent the reaction of the.
This process is also called an oxidation reaction. Although its a complex process the chemical equation is simply 4Fe 3O2 6H2O 4FeOH3. This process is also called an oxidation reaction.
The electrons lost by one element or gain by another element. An example of a synthesis reaction is the formation of water from hydrogen and oxygen. Synthesis reactions release energy in the form of heat and light so they are exothermic.
An example of a synthesis reaction is the combination of sodium Na. Iron water oxygen rust. How does knowing the reactants and help you classify a chemical reaction.
Think about the information that you needed on the previous page to determine that rusting is a synthesis reaction. After iron has reacted with an oxygen molecule the newly formed iron oxide reacts with water to yield hydrated iron oxide which is another name for rust. Then fill in the blanks to complete the lesson question below.