Peerless Incomplete Combustion Reaction Products
:max_bytes(150000):strip_icc():format(webp)/methanecombustion-58e3e6005f9b58ef7e0daa10.jpg)
The optimal acetyleneair ratio should be about 225.
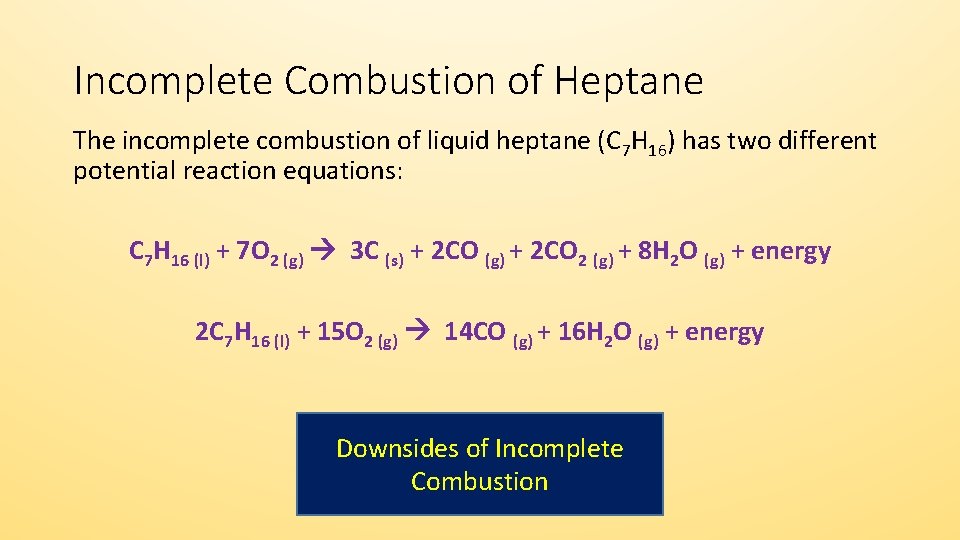
Incomplete combustion reaction products. The products of the incomplete combustion of octane C8H18 are carbon monoxide CO and water. Smoke is a byproduct of incomplete combustion. Air is 15th O 2 so you need 5 times as much air as youd need pure O 2.
Incomplete combustion due to either rich or lean burns may produce harmful combustion by-products such. We are taught that smoke poses the greatest dan-ger to our respiratory system and as long as weware our SCBA we will beprotected although still true smoke and the hot gases suspended in the smoke plume have be-come an even greater concern when it comes. Incomplete combustion can produce a variety of different byproducts depending on the fuel burned.
During incomplete combustion part of the carbon is not completely oxidized producing soot or carbon monoxide CO. Hydrocarbon oxygen carbon monoxide carbon water The carbon is released as soot. Water is still produced but carbon monoxide and carbon are produced instead of carbon dioxide.
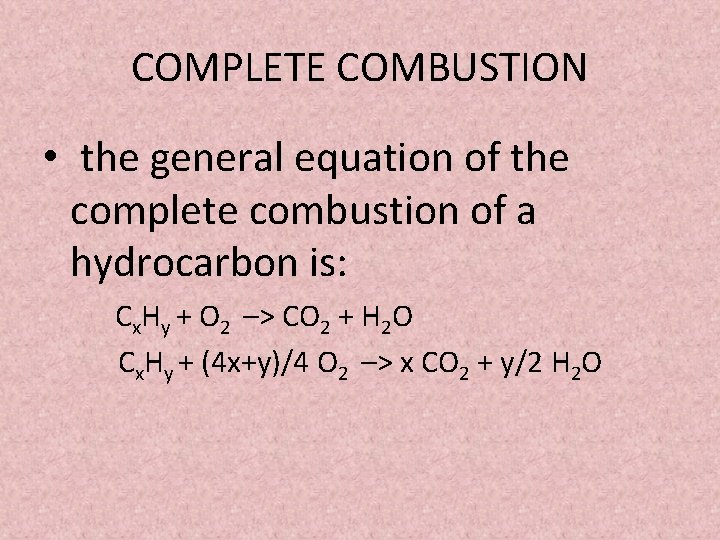
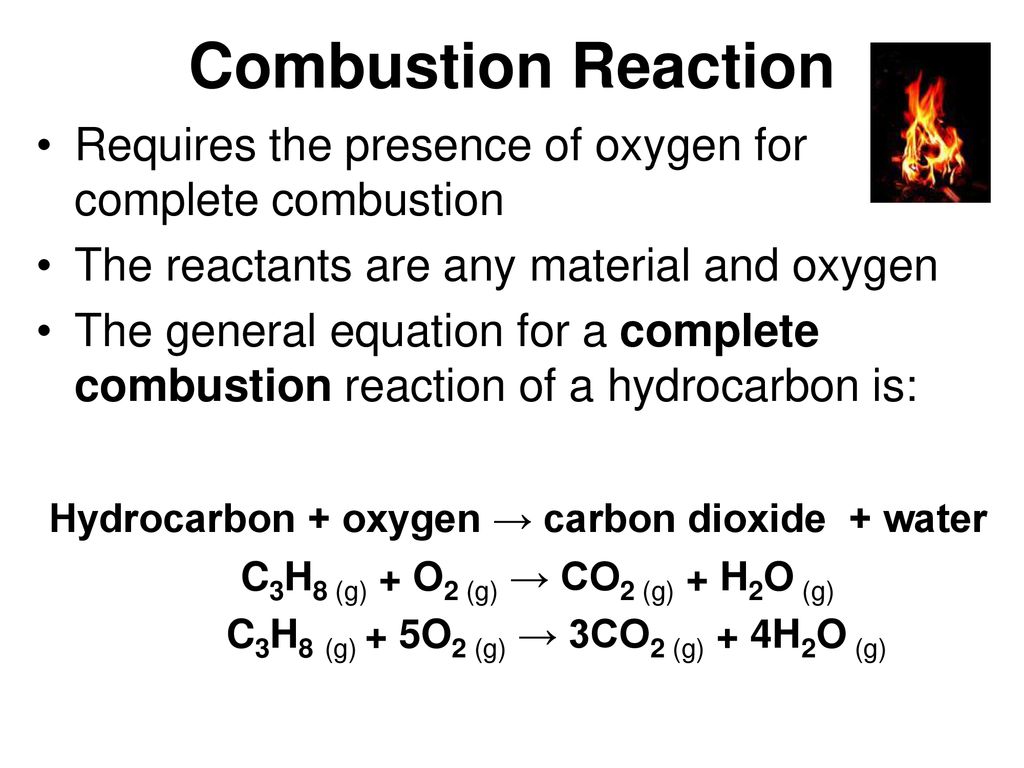
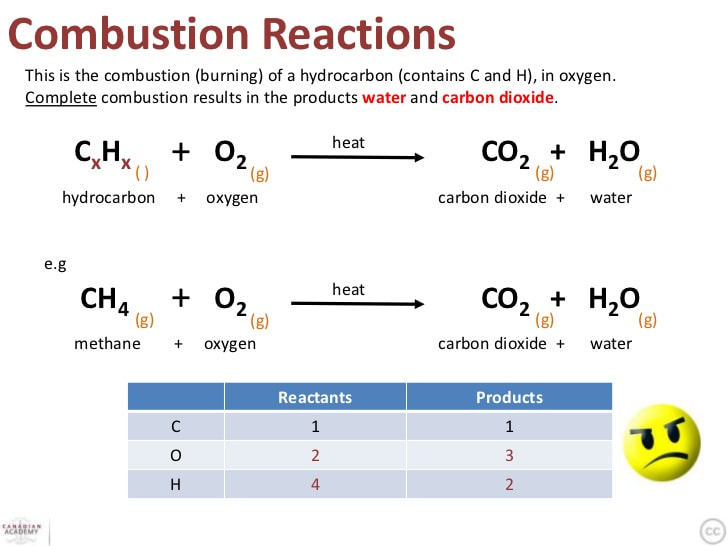
In complete combustion there is a sufficient supply of oxygen which is able to react with the burning hydrocarbon allowing the reaction products H2O and CO2 to be formed. Incomplete combustion is also a reaction between oxygen and fuel but the products are carbon monoxide water and carbon. It has been the constant companion of firefighters for years and we have taken it for granted.
In incomplete combustion there is a lack of oxygen so when heat is applied carbon monoxide is released. The complete combustion of hydrocarbons leads to carbon dioxide and water formation while incomplete combustion yields carbon dioxide carbon monoxide soot and water. What are the products in a combustion reaction.
Incomplete combustion occurs when the supply of air or oxygen is poor. Even at this ideal ration combustion products known as flue gases still occur. In complete combustion there is a sufficient supply of oxygen which is able to react with the burning hydrocarbon allowing the reaction products H2O and CO2 to be formed.