Beautiful Work How To Write An Incomplete Combustion Reaction
The following episode looks at both Complete and Incomplete Combustion reactions.
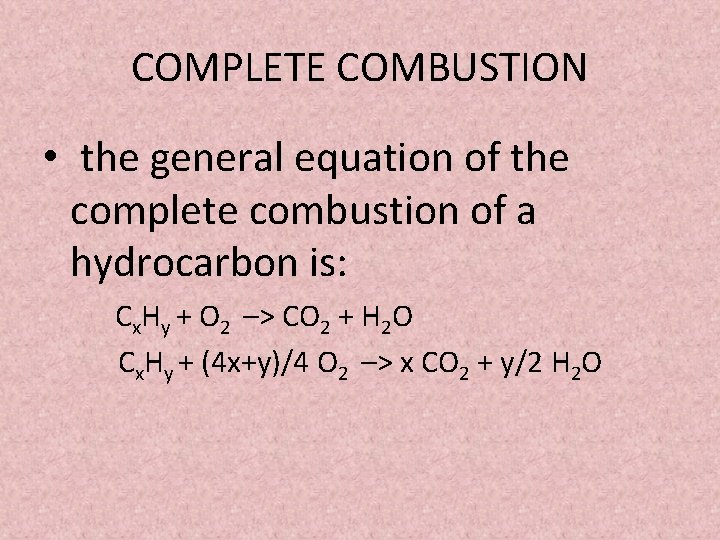
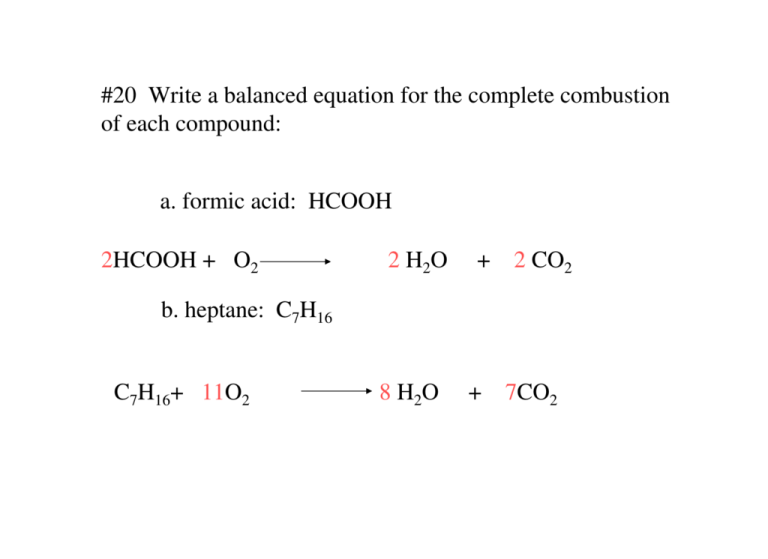
How to write an incomplete combustion reaction. A O2 AO. Recognizing the characteristics and balancing incomplete combustion reactions. The general equation for combustion is.
Complete combustion is very important in. Recognizing the characteristics of a complete combustion reaction. Magnesium is an example of an element that can undergo a combustion reaction.
The reaction of magnesium and oxygen produces a very bright light and quite a bit of heat burning at a temperature of 3100 C. How to write combustion reactions This is mostly thermal energy butHere is a quick demonstration showing the combustion of magnesium The formula for ethanol is ceC_2H_5OHMany reactants called fuels contain mostly carbon and hydrogen atoms reacting with oxygen to produce CO 2 and H 2 OCombustion is commonly called burning and the substance. The combustion process can be complete or incomplete depending on the availability of the oxygen reactant.
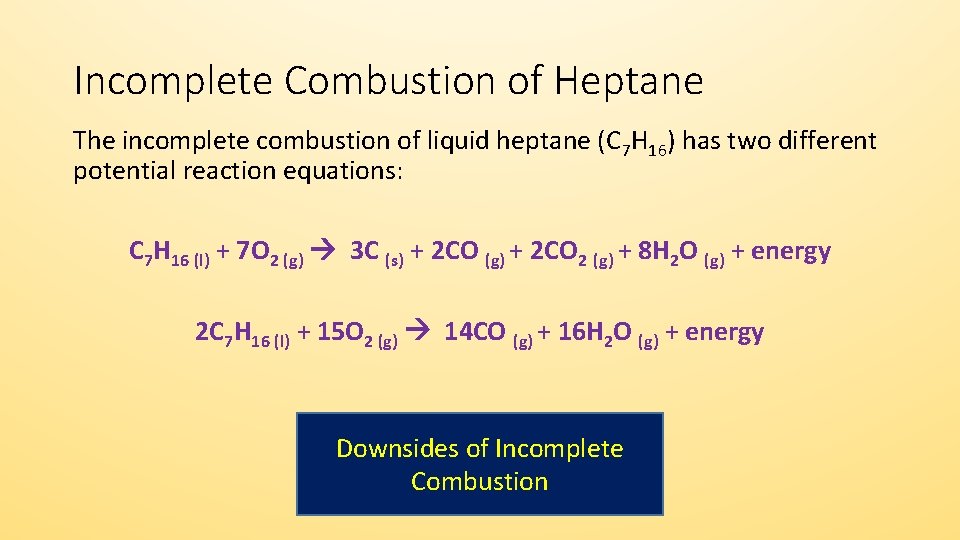
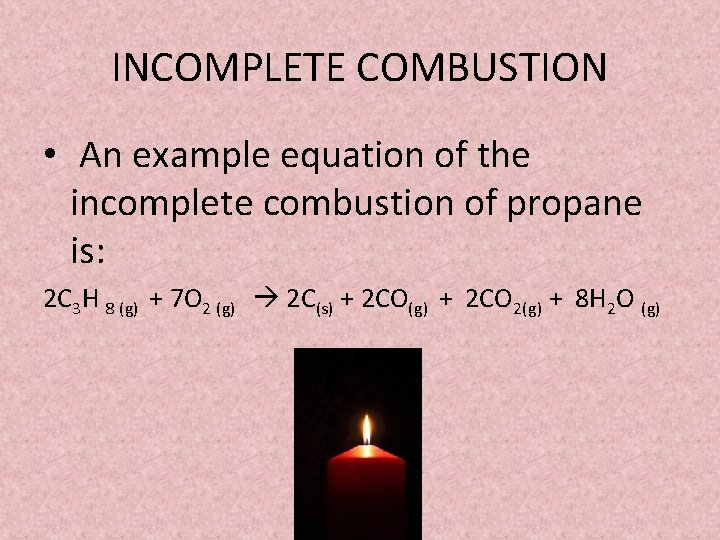
A Incomplete Combustion Equation - Multiple carbon products Hydrocarbon Oxygen Carbon Carbon monoxide water When writing equations with incomplete combustion it is advisable to include only one carbon product otherwise there will be multiple solutions to the equation. Learn to write and balance a combustion reaction. Besides the straightforward combustion reactions you have two more Boudouard reaction which favors formation of ceCO from ceCO_2 and Water-gas shift reaction.
This is mostly thermal energy but. It is an example of an exothermic reaction a reaction that releases energy to the surroundings. Before we balance the incomplete combustion of pentane lets remember first that the.
It usually occurs when a hydrocarbon reacts with oxygen to produce carbon dioxide and water. Incomplete combustion occurs when there isnt enough oxygen to allow the fuel to react completely with the oxygen to produce carbon dioxide and water and also when the combustion is quenched by a heat sink such as a solid surface or flame trap. Combustion is another name for burning.