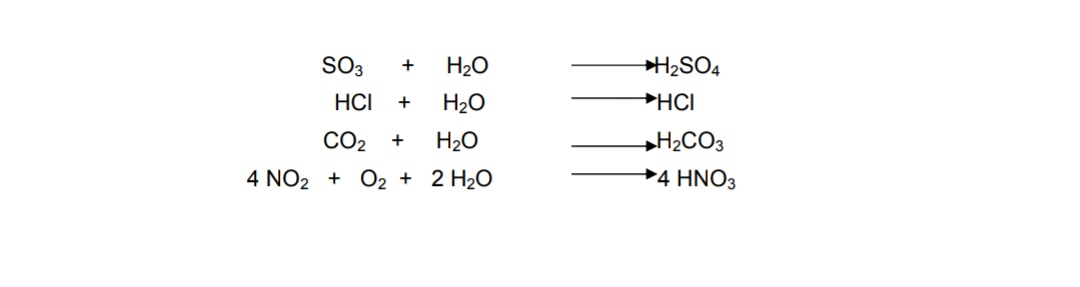Fun Acid Rain Chemical Reaction Equation

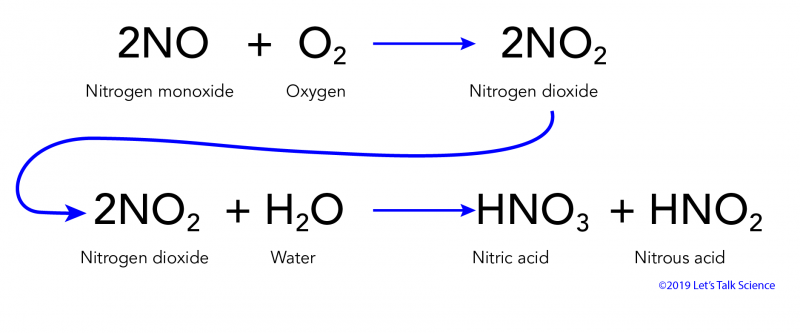
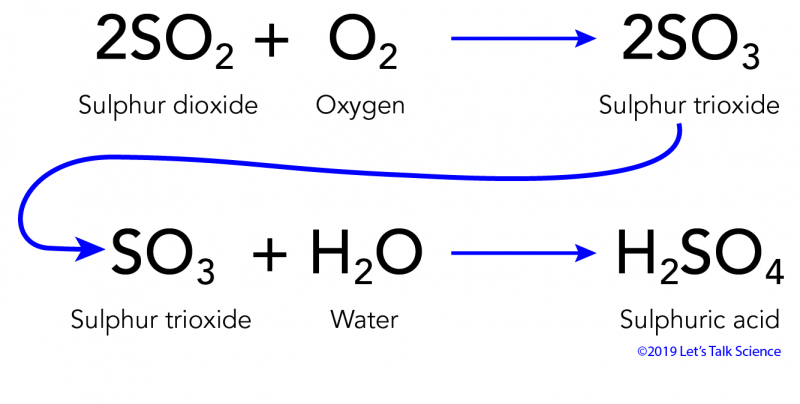
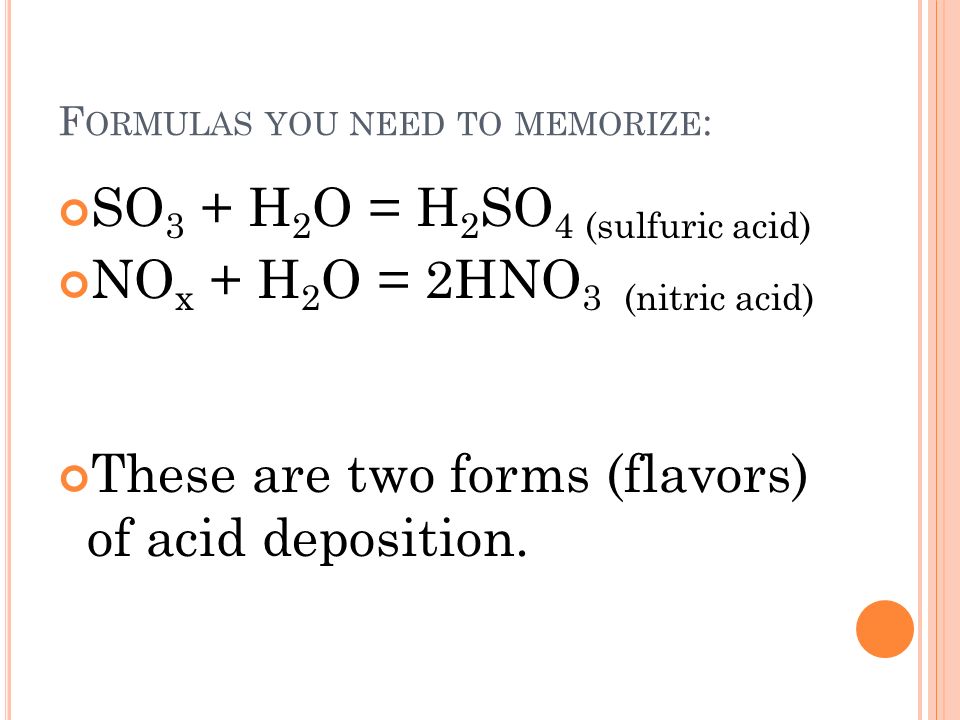
Eventually these oxides react with oxygen and water to give nitric acid and sulfuric acid.
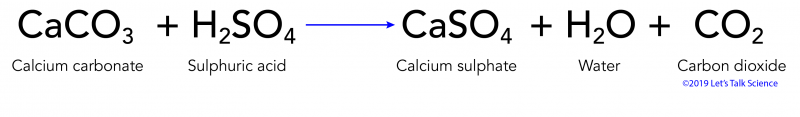
Acid rain chemical reaction equation. Acid rain is caused by nitrogen oxides and sulfur dioxide produced by both natural processes and the combustion of fossil fuels. Acids in acid rain promote the dissolution of calcium carbonate by reacting with the carbonate anion. Acid rain results when sulfur dioxide SO 2 and nitrogen oxides NO X are emitted into the atmosphere and transported by wind and air currents.
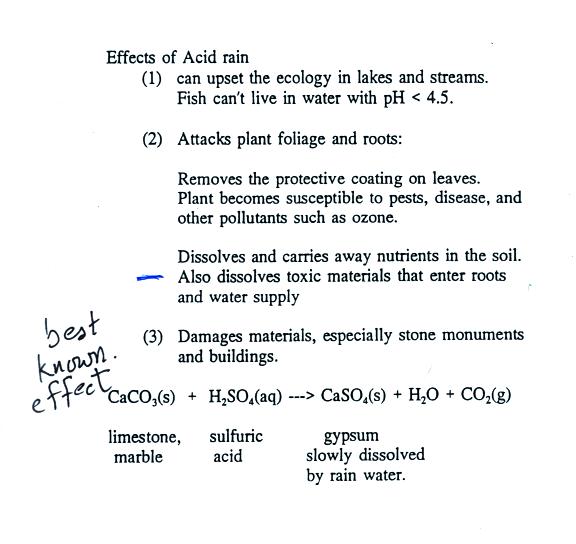
Acid rain - acid rain - Chemistry of acid deposition. Acid rain pollutes the air and corrodes buildings monuments and statues made of metals and marble. Acetone up Air.
In air NO is oxidized to nitrogen dioxide NO2 Equation 4 which in turn reacts with water to give nitric acid. Acid rain is a rain or any other form of precipitation that is unusually acidic meaning that it has elevated levels of hydrogen ions low pHIt can have harmful effects on plants aquatic animals and infrastructure. These then mix with water and other materials before falling to the ground.
Acid rain is caused by emissions of sulphur dioxide and nitrogen oxide which react with the water molecules in the atmosphere to produce acids. Be originating somewhere. In air NO is oxidized to nitrogen dioxide NO2 Equation 4 which in turn reacts with water to give nitric acid HNO3 Equation 5.
In this heavily industrialized. Metals like iron and calcium carbonate react with the acid in the rain slowly as follows. SO 2 HOH - H 2 SO 3.
The SO 2 and NO X react with water oxygen and other chemicals to form sulfuric and nitric acids. But acid rain can have pH levels lower than 43-where is the extra acidity coming from. Acid rain is rainfall whose pH is less than 56 the value typically observed due to the presence of dissolved carbon dioxide.