Fun Formation Of Rust Equation

In the absence of any one of these rusting does not occur to any significant extent.
Formation of rust equation. It is estimated that about one-seventh of all iron production goes to replace the metal lost to corrosion. In this Grade 9 Natural Sciences video lesson we will be teaching you about Reactions of Iron With Oxygen - Formation of RustWeve sourced highly-qualified. Give the equation for the formation of rust.
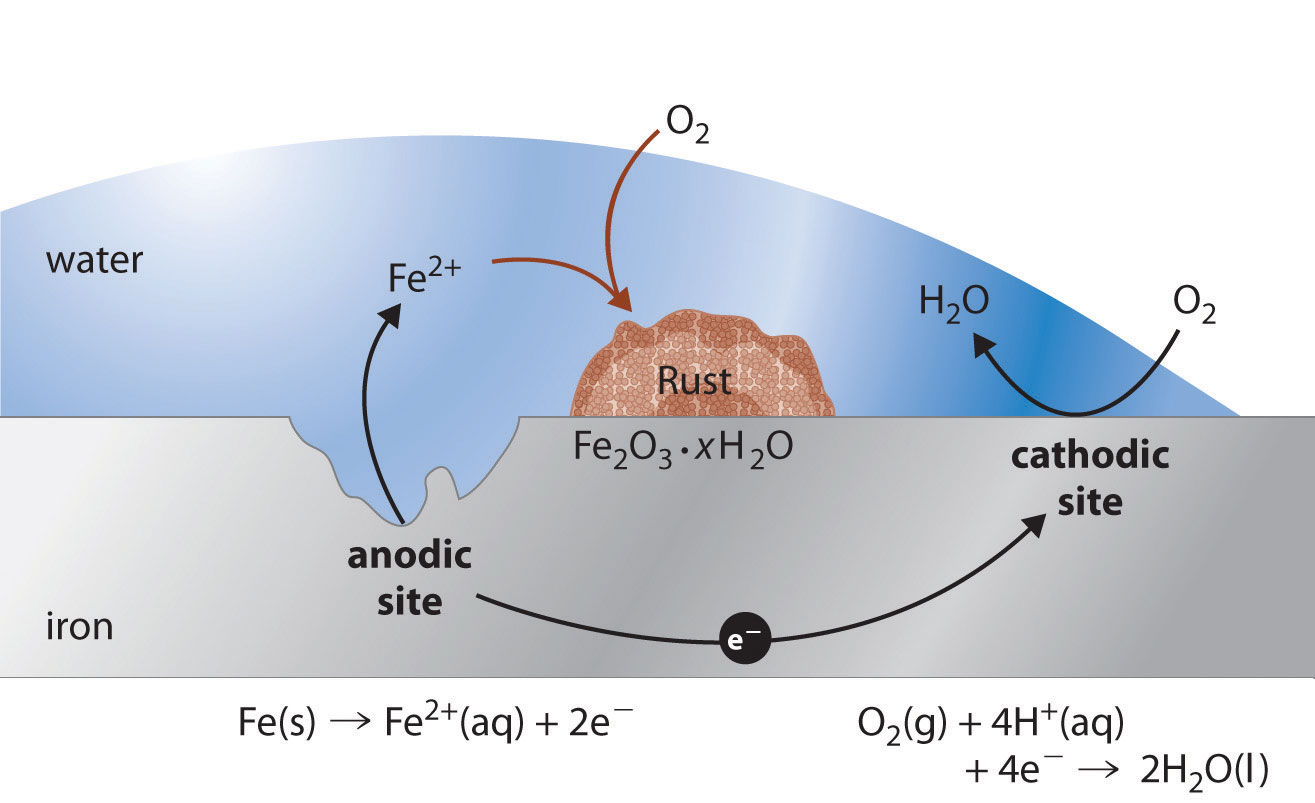
Here is the word equation for the reaction. Rust is a form. The final product in a series of chemical reactions is simplified below as- The rusting of iron formula is simply 4Fe 3O2 6H2O 4Fe OH3.
Better learning for better results. It is an electrochemical process which requires the presence of water oxygen and an electrolyte. In Chemistry some chemicals are combined to form.
The oxygen and water in air react with the metal to form the hydrated oxide. Advertisement Remove all ads. The familiar red form of rust is Fe 2 O 3 but iron has other oxidation states so it can form other colors of rust.
The chemical formula for rust is Fe2O3. The following chemical equation represents the reaction. Metal oxygen metal oxide.
These reactions are a type of process in which two or more components are combined to give other final components. Rust is formed when iron reacts with oxygen in moist air. Although its a complex process the chemical equation is simply 4Fe 3O2 6H2O 4Fe OH3.
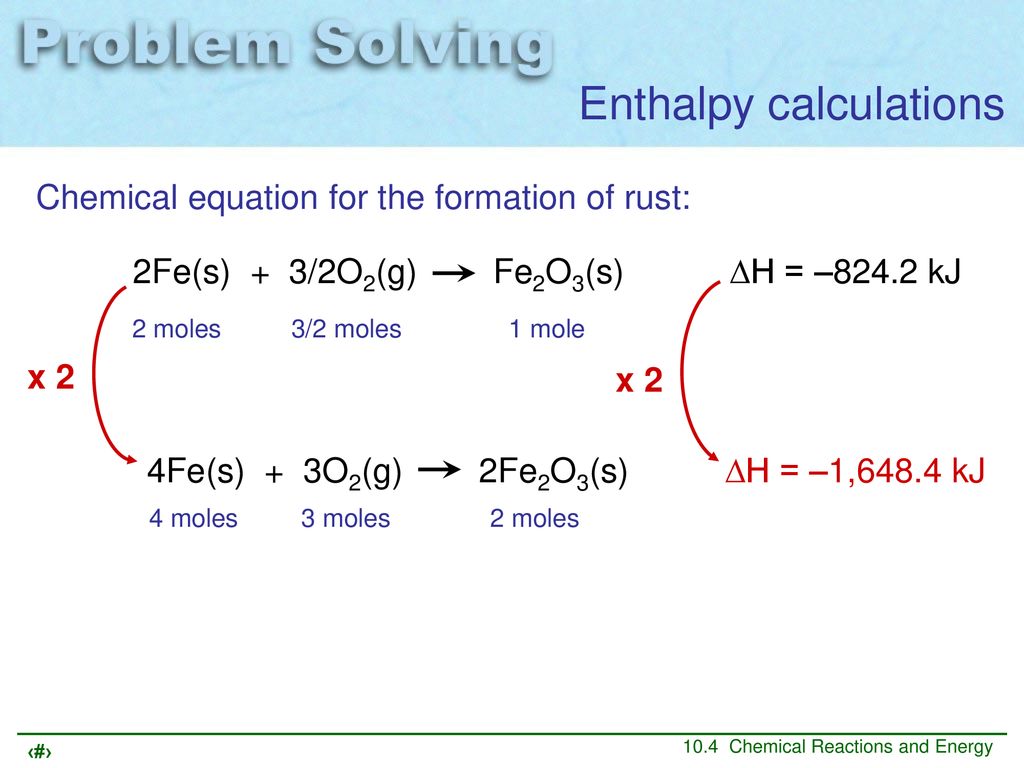







.jpg)