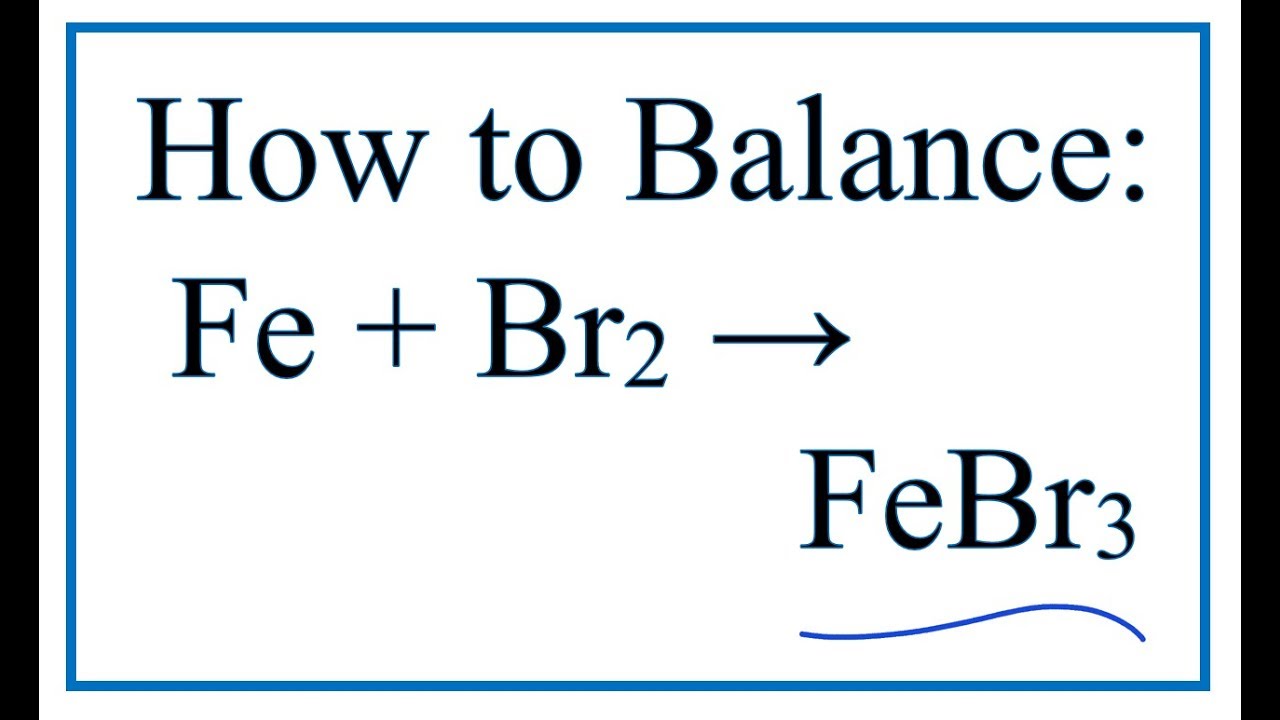Great Iron Bromide Balanced Equation

All the reactants and products are in aqueous phase.
Iron bromide balanced equation. 2Fe 3Br 2 2FeBr 3 Check the balance Iron react with bromine to produce iron III bromide. CHEMICAL EQUATIONS II. Fe Cl 2 FeCl 3.
This means that when you mix solutions of Fe NO33 and KBr - no reaction takes place. Iron reacts with bromine gas according to the following balanced chemical equation. Also known as ferric bromide this red-brown odorless compound is used as a Lewis acid catalyst in the halogenation of aromatic compounds.
2 CoBr 3 aq 3 K 2 Saq Co 2 S 3 s 6 KBr aq Total Ionic. It dissolves in water to give acidic solutions. 2 Co 3 aq 6 Br aq 6 K aq 3 S 2- aq Co.
The reaction produces solid ironIII sulfide and aqueous hydrogen bromide. What is the percent yield of iron III bromide if 49019 g. Iron III bromide is the chemical compound with the formula FeBr 3.
Iron reacts with oxygen gas to produce Iron III oxide. FeBr 3 2H 2 O Fe OH 2 Br 2HBr Check the balance Iron III bromide react with water to produce iron III dihydroxide-bromide and hydrogen bromide. Direct link to this balanced equation.
Only change the coefficients these are the numbers in front substances. Propane C 3H8 reacts with oxygen gas to produce carbon dioxide and water. A reaction occurs when hydrosulfuric acid leftmathrmH_2 mathrmSright is mixed with an aqueous solution of ironIII bromide.