Simple Example Of Thermal Decomposition Reaction
Example of Decomposition Reaction H2CO3 aq CO2 g H2O l This is an example of decomposition reaction in which carbonic acid H 2 CO 3 is breakdown into carbon dioxide and water.
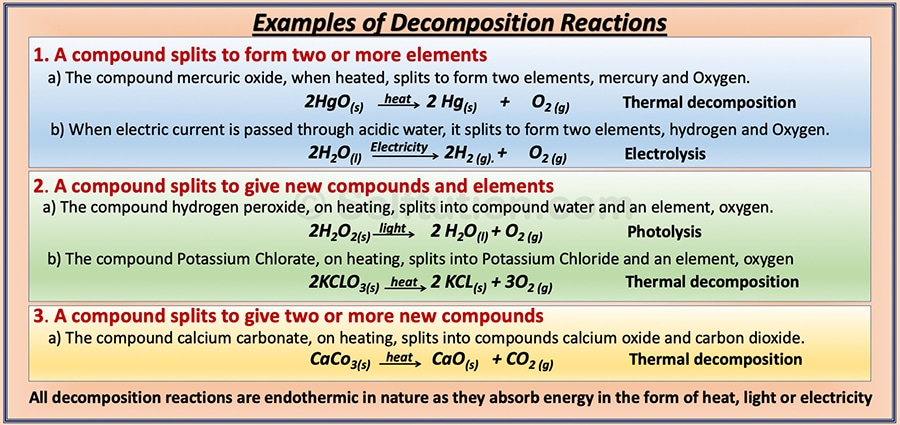
Example of thermal decomposition reaction. Thermal decomposition reaction. CaCO3 CaO CO2. When silver chloride is kept in sunlight it decomposes to form silver and chlorine.
In other words a thermal decomposition reaction requires energy to be supplied to the reactants in the form of heat. Such reactions are generally endothermic since energy is required to break the chemical bonds and separate the constituent elements. Zinc carbonate on heating decomposes to form Zinc oxide and carbon dioxide.
The temperature at which this chemical reaction initiates is the. 1 A solution of substance X is used for white-washing. CaCO3s heat CaOs CO2g CaCO3s-Calcium carbonate.
In simpler words a thermal decomposition reaction needs energy to be supplied to the reactants in the form of heat. CuCO 3 CuO CO 2. For example copper carbonate breaks down easily when it is heated.
Photo chemical decomposition reaction. Many metal carbonates can take part in thermal decomposition reactions. Question Papers 10.
This type of reaction is called thermal decomposition. In a decomposition reaction a single reactant breaks down into two or more simpler products. Calcium carbonate decomposes to form calcium oxide and carbon dioxide.









.PNG)