Stunning Rusting Of Nails Evidence Of Chemical Change

Is a nail rusting a physical or chemical change.
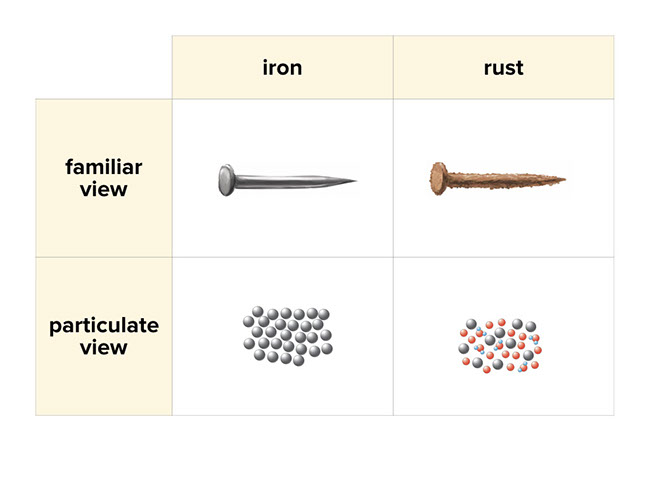
Rusting of nails evidence of chemical change. These would be an example of chemical change because they undergo one of the 4 criteria. The following can indicate that a chemical change. A new substance is formed.
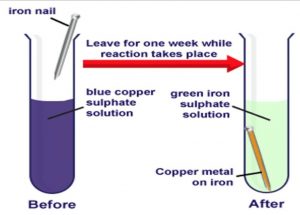
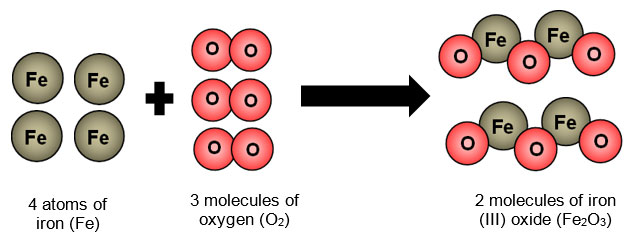
Chemical reaction taking place during rusting is shown below. No the rusting of iron is a chemical change because it is two substances reacting together to make a new substance. Why are burning candles and rusting nails examples of chemical change.
This rust is formed from a redox reaction between oxygen and iron in an environment containing water such as air containing high levels of moisture. Aluminium Foil No change or evidence of corrosion was observed. Rust is the common name for iron oxideThe most familiar form of rust is the reddish coating that forms flakes on iron and steel Fe 2 O 3 but rust also comes in other colors including yellow brown orange and even greenThe different colors reflect various chemical compositions of rust.
Rusting is an example of a chemical change. QUESTION 8 What evidence of a chemical reaction you might see when rusting occurs in an iron nail. The evidence of chemical reaction in the rusting of an iron nail is a color change.
Burning a sugar cube is a chemical change. Why are burning candles and rusting nails examples of chemical change. It results in the formation of Iron Oxide which is an entirely new substance.
Applying hydrogen peroxide on an open wound 7-photosynthesis 8. Heating a sugar 4. How does a combination reaction differ from a decomposition reaction.