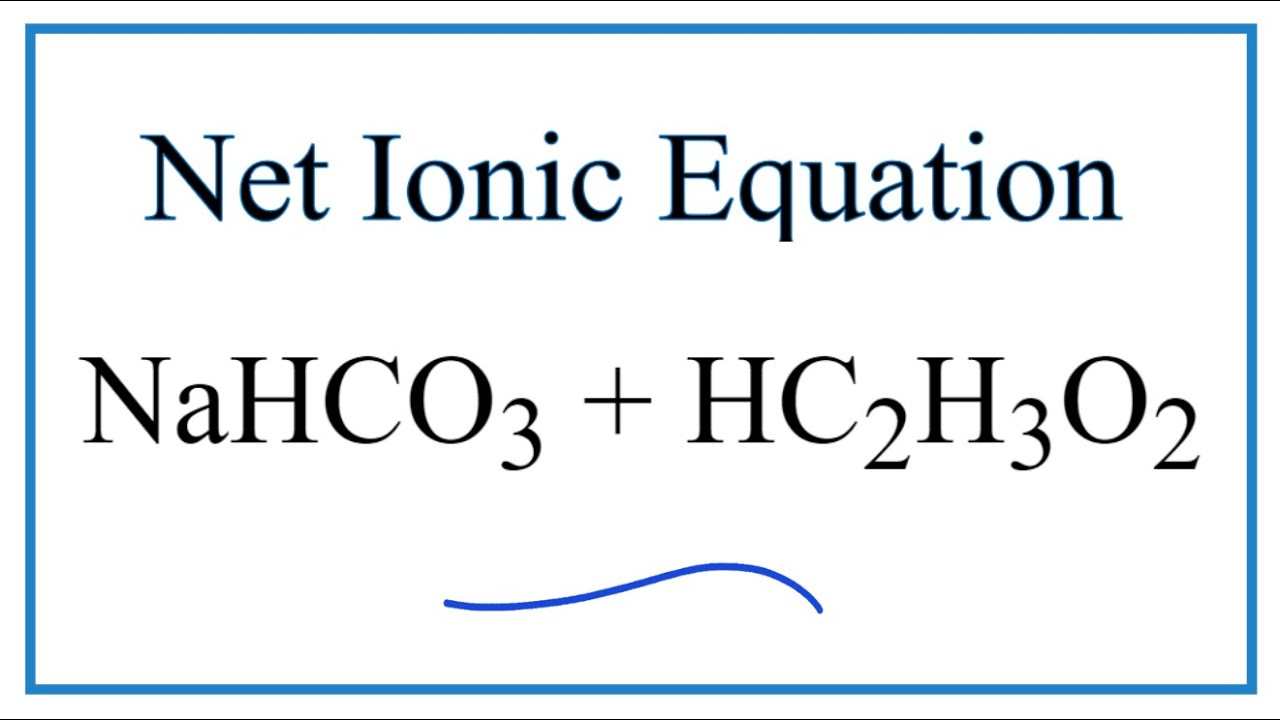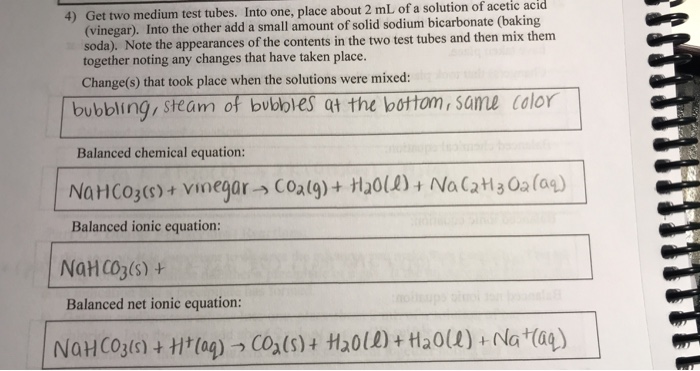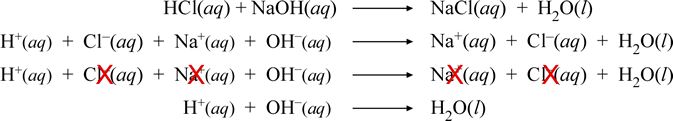Exemplary Baking Soda And Vinegar Net Ionic Equation
When baking soda is added to vinegar the resulting reaction produces a tremendous amount of gas as shown in this video.
Baking soda and vinegar net ionic equation. The answer I got was H aq HCO3- aq H2O l CO2 g but apparently. For fresh cabbage juice 4-5 leaves of fresh red cabbage water 500 mL denatured alcohol blender funnel cheesecloth or paper towel 600 mL beaker. Asked Jun 29.
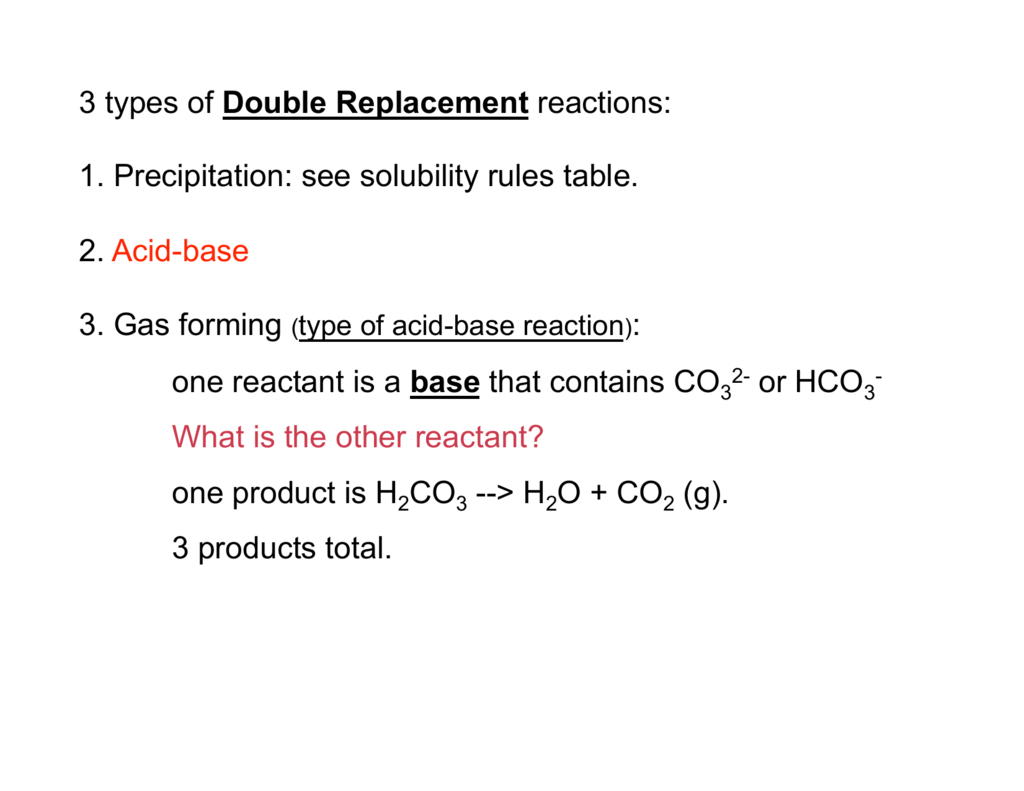
NaHCO3aq CH3COOHaq CO2g H2Ol CH3COONaaq In the first 2 flasks the limiting reagent is the baking soda. Vinegar ammonia baking soda lemon juice milk of magnesia clear soda aspirin vitamin C tablet etc. BAKING SODA AND VINEGAR.
Write the complete and net ionic equations with phases for the reaction that occurred placing a piece of MAGNESIUM IN VINAGER. It explains how to write the net ionic equation of the reaction betwee. The equilibrium shifts tow.
When vinegar CH3COOH and baking soda NaHCO3 react the following reaction occurs. Tall cylinder or 125 mL erlenmeyer flask. Chemical Equation Baking Soda And Vinegar.
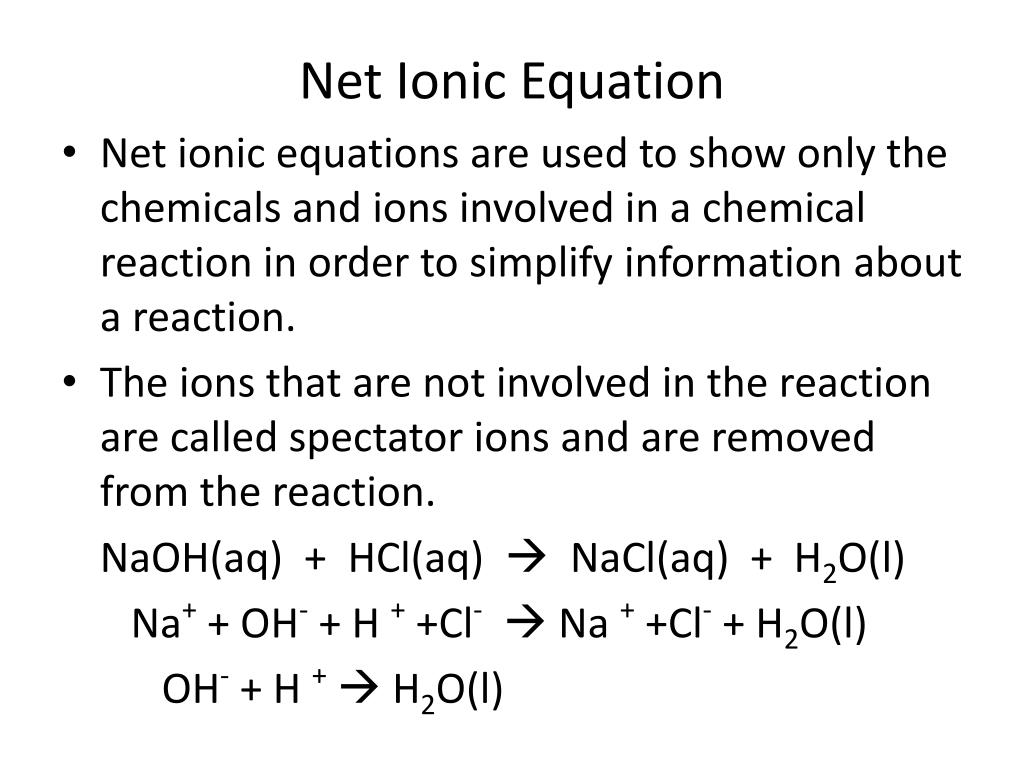
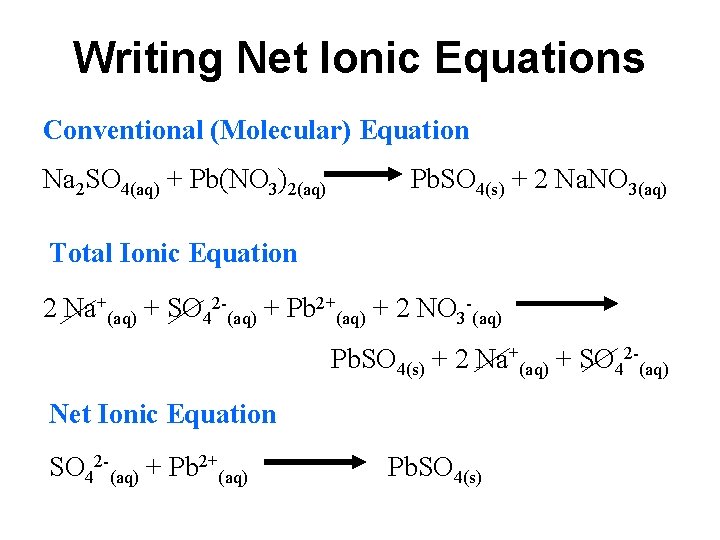
The major components of vinegar are water H2O solvent and acetic acid HC2H3O 2. Na HCO- 3 H NO- 3 Na NO- 3 CO2 H2O This gives the net ionic equation HCO- 3 H CO2 H2O. _____ Classify the type of reaction that occurred in each case or types as a reaction may fall under more than one category.
The overall chemical reaction between baking soda sodium bicarbonate and vinegar weak acetic acid is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas liquid water sodium ions and acetate ions. The reaction proceeds in two steps. You may know that vinegar aqueous acetic acid and baking soda sodium bicarbonate react to form a gas that can be used.