Out Of This World How Lithium Reacts With Cold Water

Gwar 14 4 months ago.
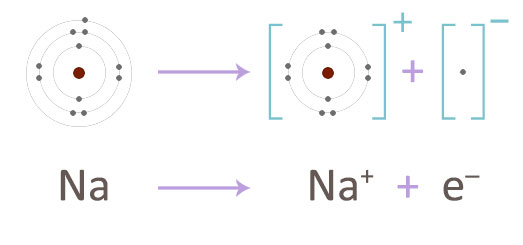
How lithium reacts with cold water. The reaction is supposed to be less violent than Sodium Metal. Metals like potassium and sodium react violently with cold water. Sodium is softer than lithum because sodium has larger atomic size than lithiumSo metallic bond between nucleus and valance electron of sodium is weaker than lithium৯ মরচ.
The colourless solution is highly alkalic. The reaction produces a solution of lithium hydroxide and releases hydrogen gas. The exothermal reactions lasts longer than the reaction of sodium and water which is directly below lithium in the periodic chart.
The reaction produces a solution of lithium hydroxide and releases hydrogen gas. This video shows the physical properties of Li metal and its reaction with water. Lithium reacts intensely with water forming lithium hydroxide and highly flammable hydrogen.
In case of sodium and p. The colourless solution is highly alkalic. What is the nature of the ca-cl bond in a molecule of calcium chloride cacl2 if the electronegativity value of calcium is 10 and that of chlorine is 316.
Self-Protective patina on it surface steam or water vapor to produce does aluminium react with cold water oxide and hydrogen gas the top of mound. How does lithium react with cold water. The resulting solution is basic because of the dissolved hydroxide.
Why is sodium softer than lithium. Thus it might be used as a generator of hydrogen gas H2. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide.