Recommendation Balanced Chemical Equation For Combustion Of Methane
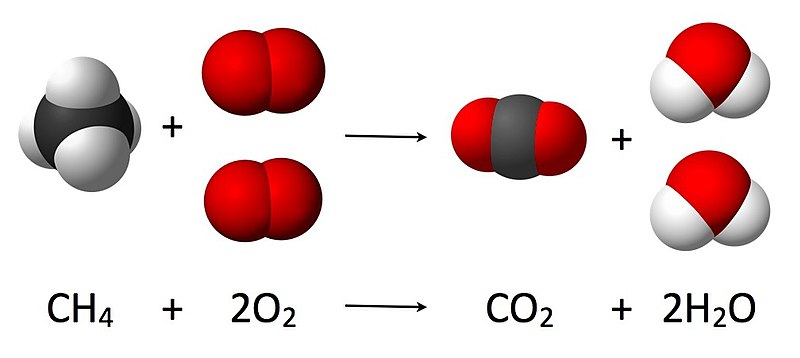
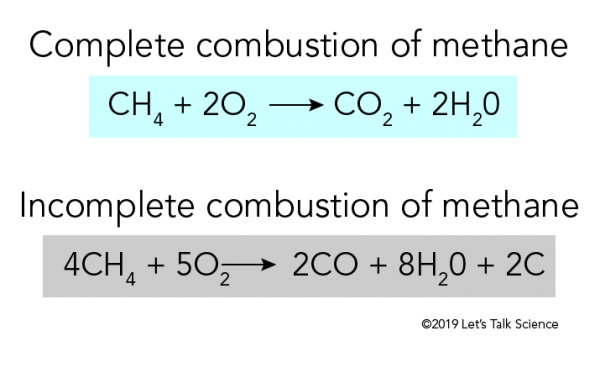
Combustion of methane CH4 in abundance of oxygen perfect combustion can follow the below reaction producing only carbon dioxide CO2 water H20 and energy.
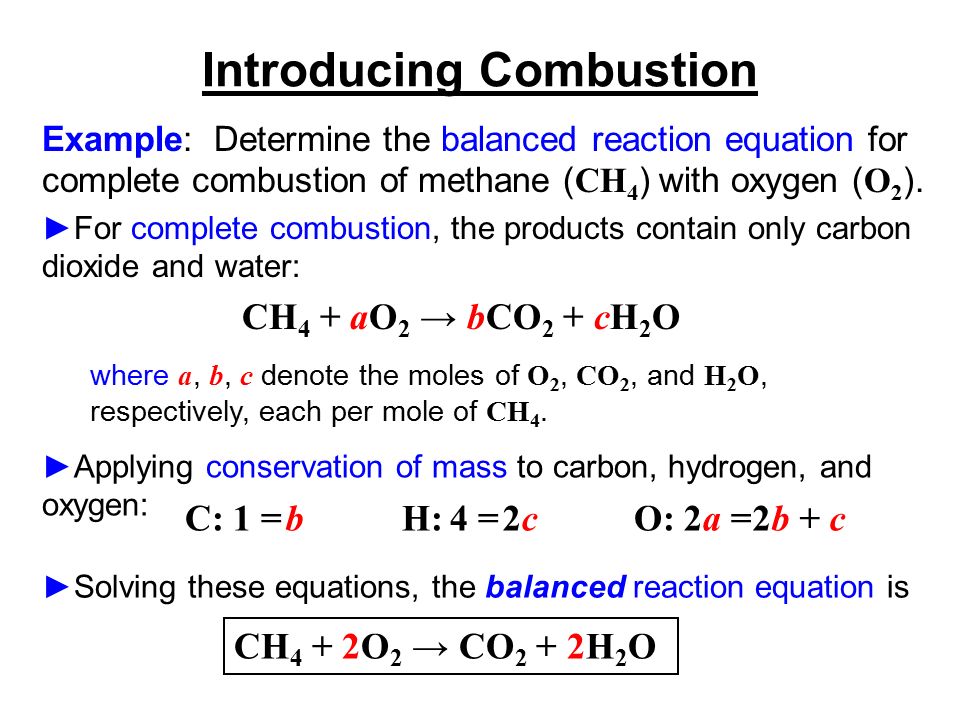
Balanced chemical equation for combustion of methane. One gram of methane gas reacts with two grams of dioxygen gas producing one gram of carbon dioxide gas and two grams of gaseous water. Suppose 0150 kg of methane are burned in air at a pressure of exactly 1 atm and a temperature of 200 C. A balanced equation for combustion of methane is given below.
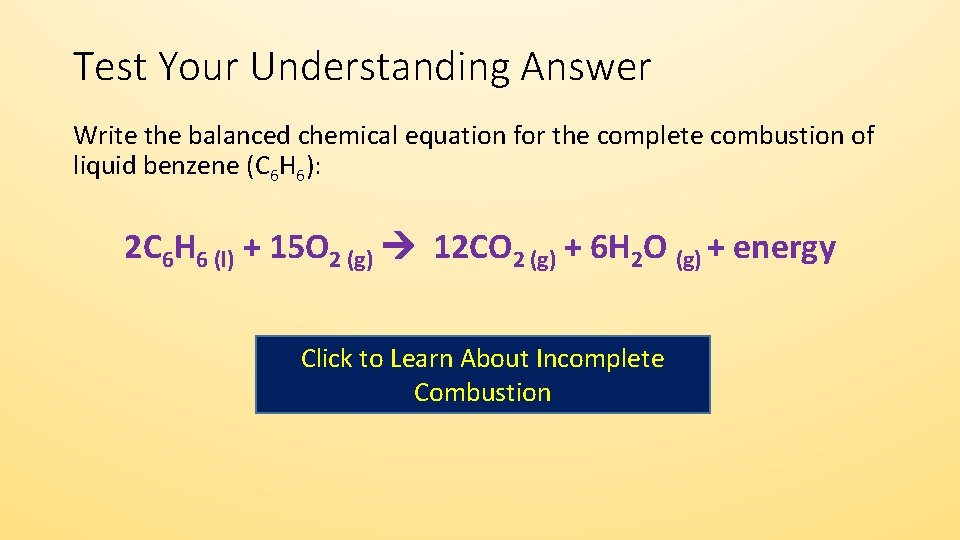
Once again start with the balanced chemical equation for the combustion of methane CH_4 CH_4 2O_2 - CO_2 2H_2O You have a 12 between methane and water this means that the number of moles of water produced must be twice the number of moles of methen that reacted. The combustion of liquid methanol CH3OH C H 3 O H in the presence of excess oxygen gas O2 O 2 to form gaseous carbon dioxide CO2 C O 2 and liquid water H2O H 2 O is shown below. Calculates the moles of air fed to a reactor and the composition of the stack gas for the complete combustion of methaneMade by faculty at Lafayette College.
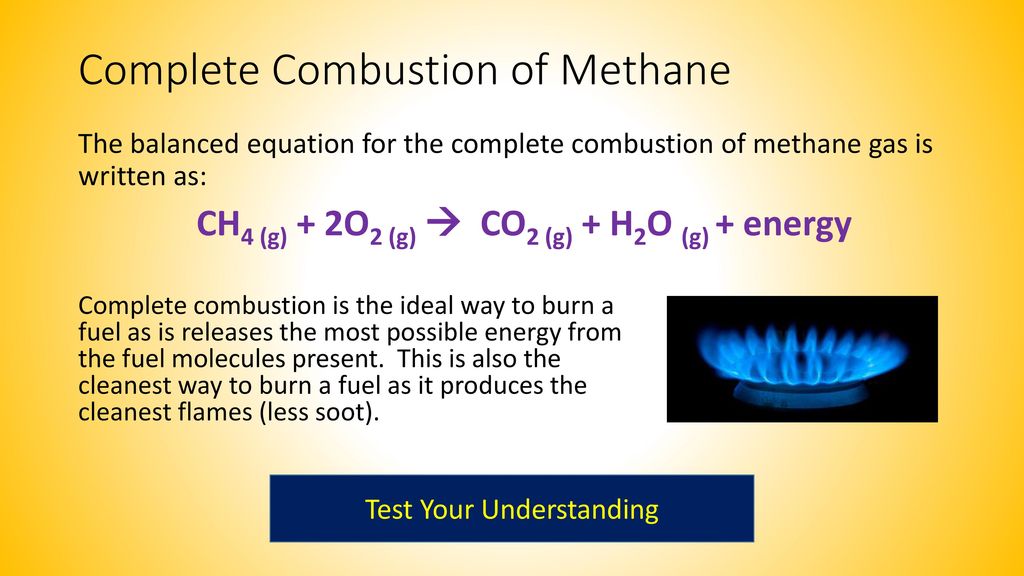
The unbalanced equation for this reaction is CH4g O2g CO2g H2Og This type of reaction is. The combustion of methane gas heats a pot on a stove. Three different liquids are used in.
CH4 2 O2 excess. The balanced chemical equation for the combustion of methane is. The balanced chemical equation for the combustion of methane is.
The balance equation for the complete combustion of ethane is. Additionally what are the products for the combustion of methane ch4. CH4 g 2 O2 g CO2 g 2 H2O g Which of the following statements concerning this chemical equation isare correct.
The combustion of methane CH4 produces carbon dioxide CO2 and water. The balanced chemical equation for the combustion of methane is. When methane CH4 burns it reacts with oxygen gas to produce carbon dioxide and water.