Fine Beautiful What Is The Equation For The Combustion Of Propane
Explained Process of Combustion of Propane.
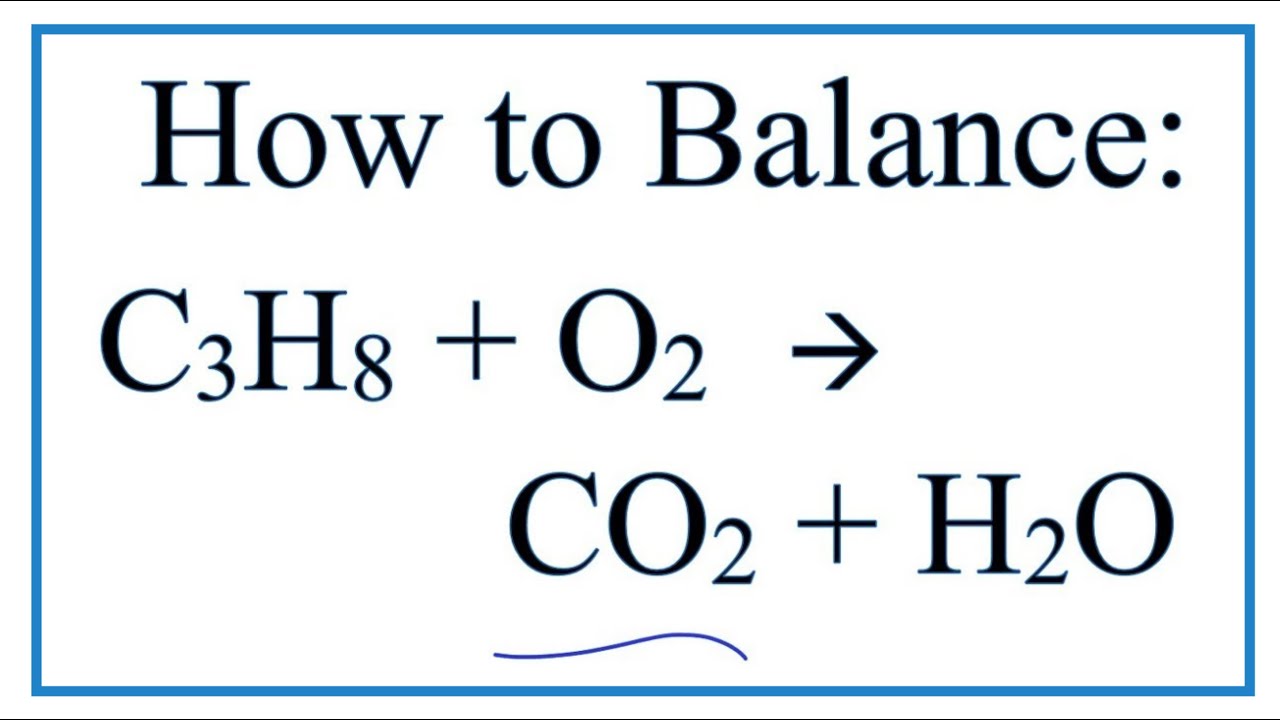
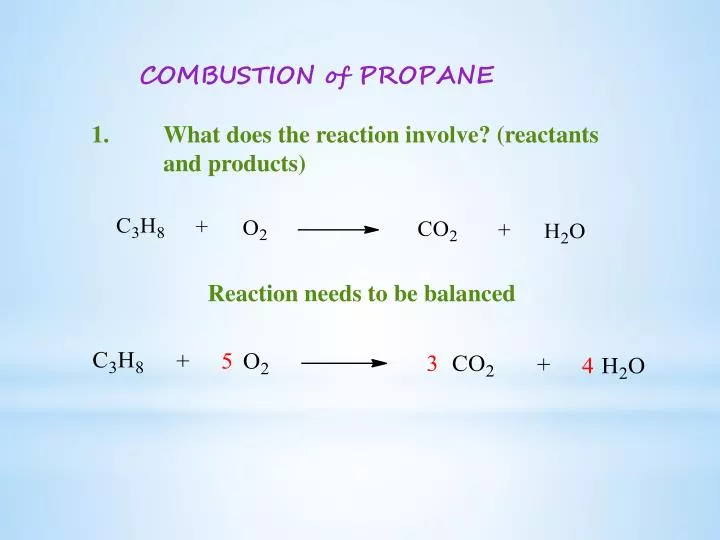
What is the equation for the combustion of propane. Propane C3H8 Combustion reaction of propane C3H8 5O2 ---- 3CO2 4H2O 1 mol of propane needs 5 mols of O2 Air contain 21 oxygen and 79 n View the. This problem has been solved. Propane oxygen carbon dioxide water C3H8 5O2 3CO2 4H2O.
See full answer below. As a type of hydrocarbon it can undergo hydrocarbon combustion which gives off heat. INCOMPLETE COMBUSTION An example equation of the incomplete combustion of propane is.
2 C3H8 9 O2 4 CO2 2 CO 8 H2O Heat. Write the equation of propane-air combustion and determine the stoichiometric air-fuel ratio. But it also produces carbon monoxide.
1- Firstly the carbon in the hydrocarbon propane oxidizes with oxygen to produce carbon dioxide. If not enough oxygen is present for complete combustion incomplete combustion occurs. See the answer See the answer See the answer done loading.
Given the equation ceC3H8 5O2 - 3CO2 4H2O and that enthalpies of formation for ceH2O l is pu-2853 kJmol and ceCO2 g is pu-3935 kJmol and the enthalpy of combustion for the reaction is pu-22201 kJmol I need to find the heat of formation of propane. The required equation for the combustion of propane is given as. Balancing chemical reaction balancing combustion of propanec3h8o2--co2h2o.
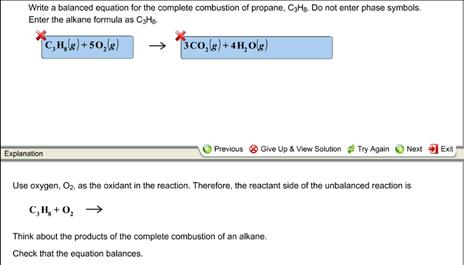
Propane is an alkane with the chemical formula C 3 H 8. Propane is one of the hydrocarbon components of raw natural gas which is a type of fossil fuel. Eq C_3 H_8left g right O_2left g right to C O_2left g right.