Brilliant Butane And Oxygen Gas Balanced Equation

Butane is a gas at room temperature and pressure.
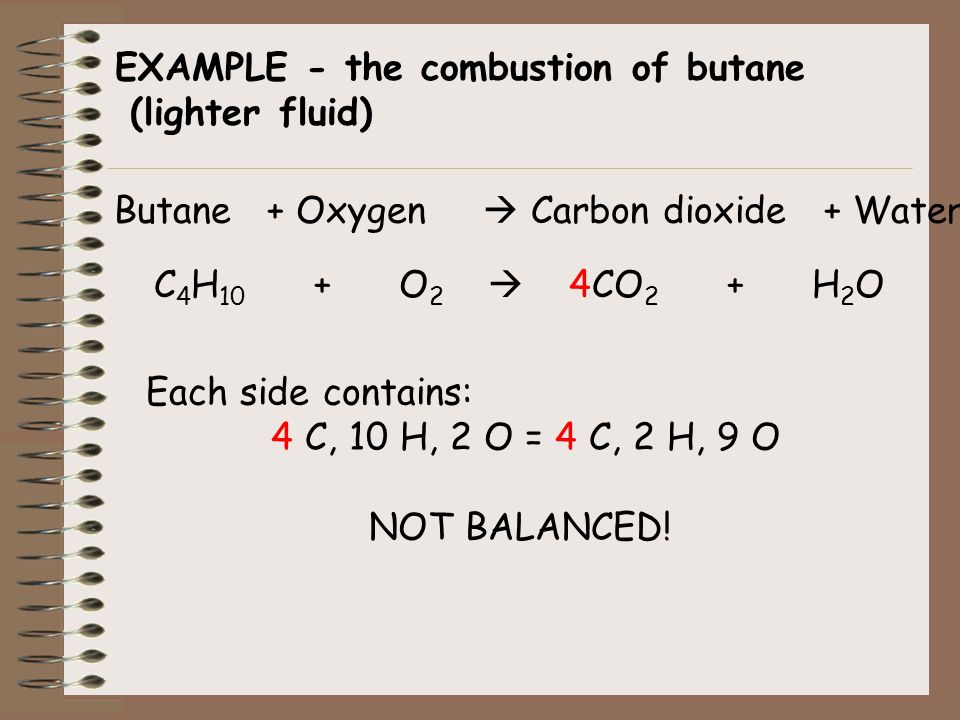
Butane and oxygen gas balanced equation. Carbon dioxide and water are produced. The balanced equation for this reaction is. M 232 gm of Butane will react Molar mass of Oxygen 32 gmol.
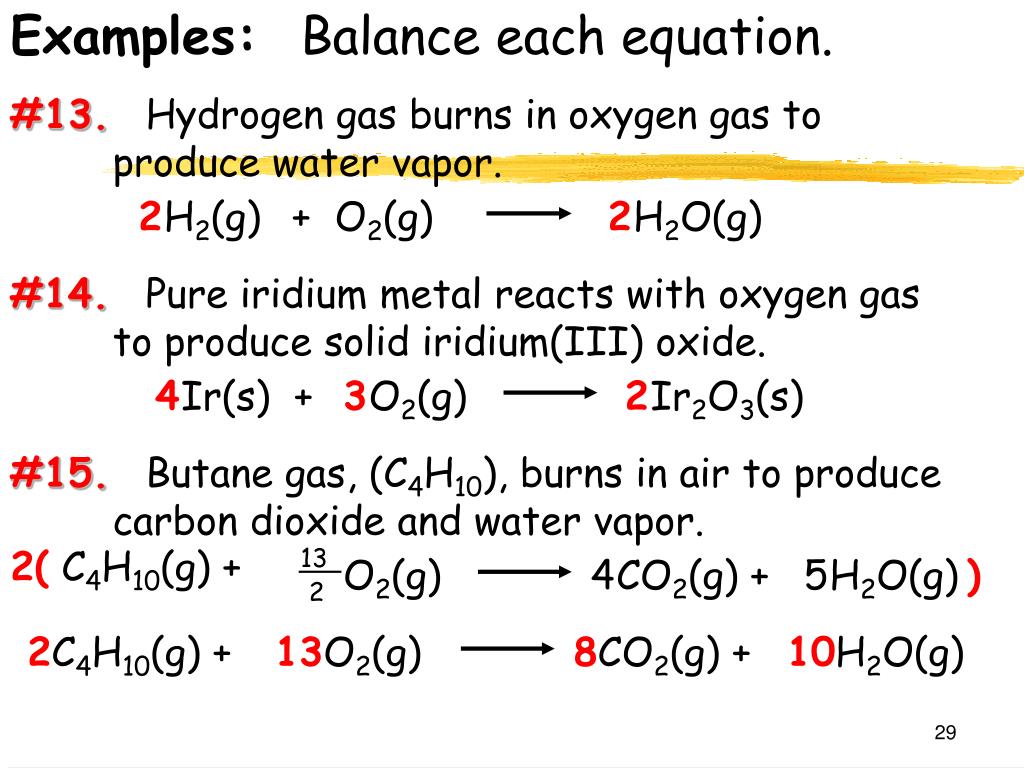
2C4H10 1302 8CO2 10H2O Energy How Many Moles Of Oxygen Gas Are Required In The Combustion Of 5 Moles Of Butane. Write a balanced chemical equation for carbon dioxide reacting with hydrogen gas H2 to produce methane and water. 2 C4H10 g 13 O2 g 8 CO2 g 10 H2O g If 8 moles of butane C4H10 react The reaction consumes.
The chemical formula of butane is C4H10. Give the complete and balanced chemical equation for the reaction between butane eqC_4H_10 eq and oxygen. 2C4H10g13O2g8CO2g10H2Ol At 100 atm and 23 C what is the.
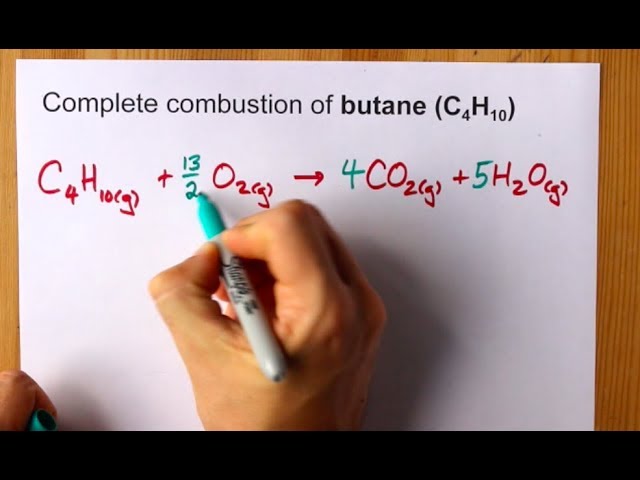
Write a balanced equation for this reaction. The balanced equation for this reaction is. Complete combustion does NOT give carbon monoxide or sootCheck me out.
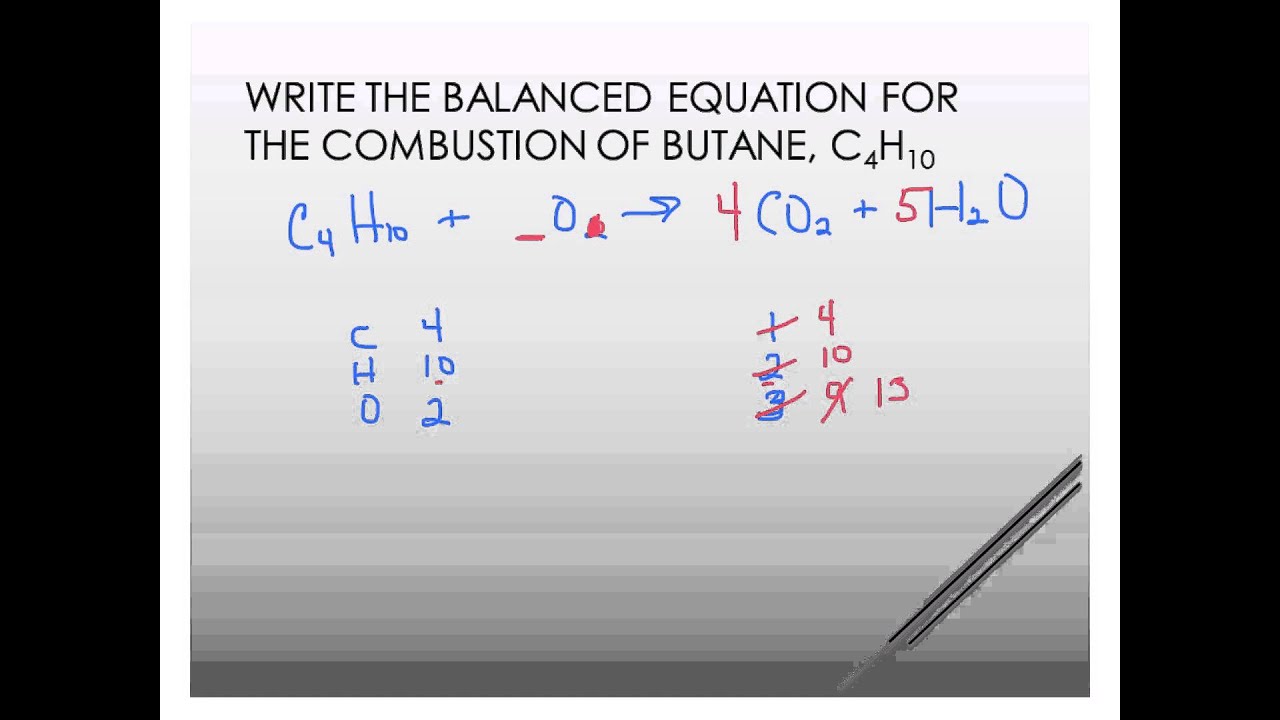
The combustion of butane in oxygen produces carbon dioxide and water. When butane C4H10 reacts with oxygen. An explanation of how to balance the equation C4H10 O2 CO2 H2O and the correct coefficients for each substance in the equationTo balance C4H10 O2.
Butane gas burns in oxygen to form carbondioxide and water. First balance the equation below CH3 CH22CH3O2CO2H2O 1. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water.