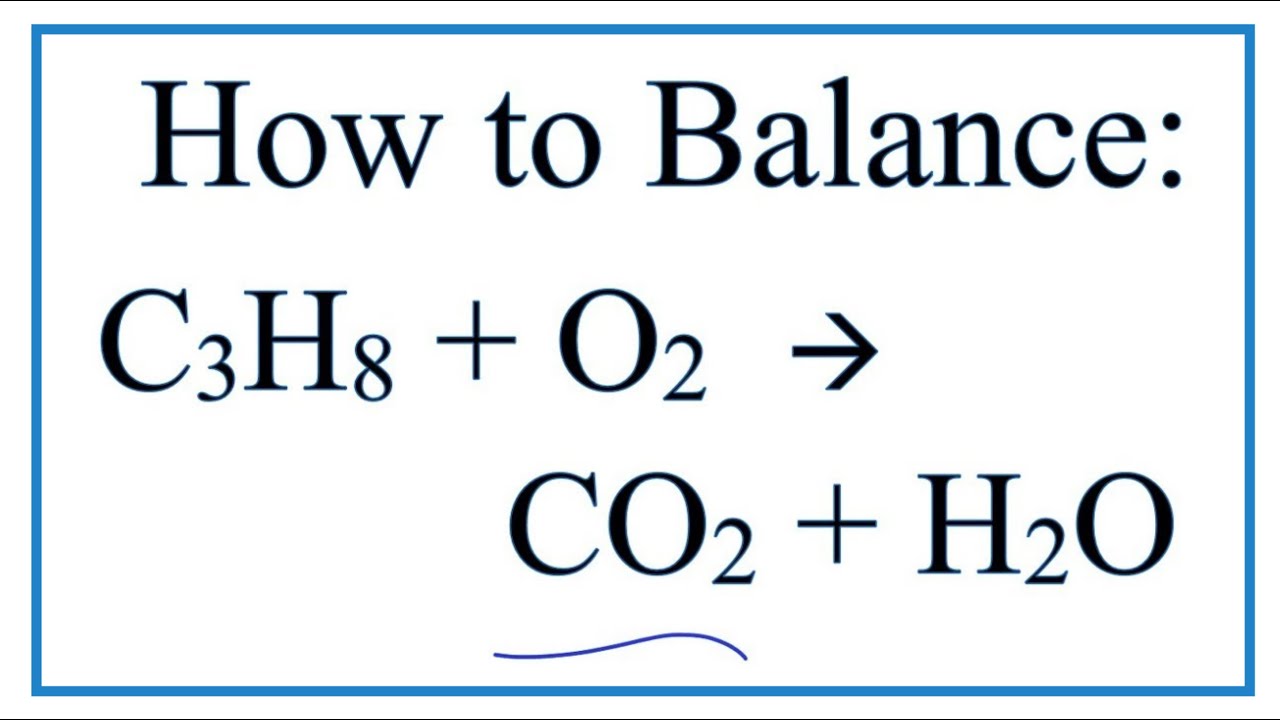
Peerless Propane Reaction With Oxygen
This reaction is an redox reaction because the carbon is oxidized while the oxygen is.
Propane reaction with oxygen. A mixture of propane and butane is burned with pure oxygen. Fuels are substances that react with oxygen to release useful energy which consists of mostly heat and light. 4 Explained Process of Combustion of Propane.
When propane burns it reacts with the oxygen in the air. If this is the case then you only need to vary the amount of oxygen. In a particular experiment 380 grams of carbon dioxide are produced from the reaction of 2205 grams of propane.
This may be simply be for practical reasons. Solution for ow many grams of Oxygen gas will completely react with 38 moles of Propane. Molecules are produced when 17 moles of.
In the presence of excess oxygen propane burns to form water and carbon dioxide. On a practical level your instructor may be working off the assumption that the reaction is first order in propane r a t e k p r o p a n e X 1 o x y g e n X. The oxidation of a hydrocarbon such as propane is a combustion reaction produces carbon dioxide and water.
Propane is a flammable naturally occurring hydrocarbon gas that is able to react with oxygen to be used as a fuel. The combustion products contain 474 mole H 2 O. Propane filled balloons are cool but not as powerful as the right ratio of oxygen and propane combined into an explosive balloonIts amazing the difference t.
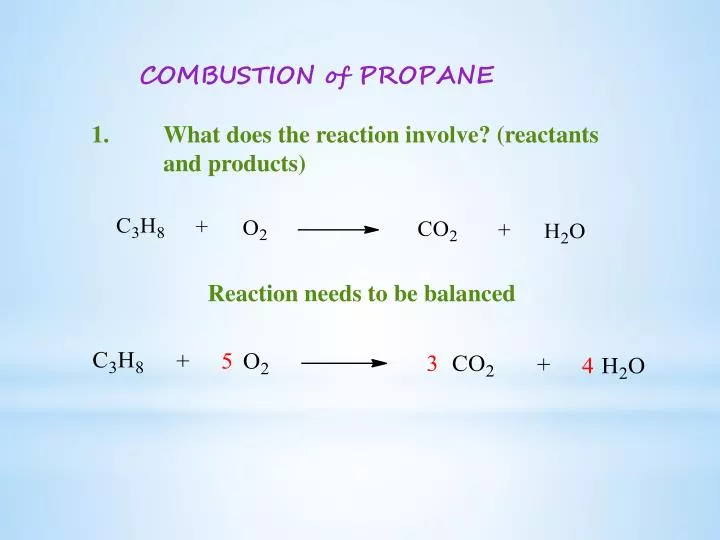
Propane gas reacts with oxygen according to this balanced equation. A How many moles of water form when 500L of propane gas completely reacts. The torch head is gaffed to the table top with enough slack to turn the gas valves.