First Class Composition Of Acid Rain
Check out my original music on your preferred platform at.
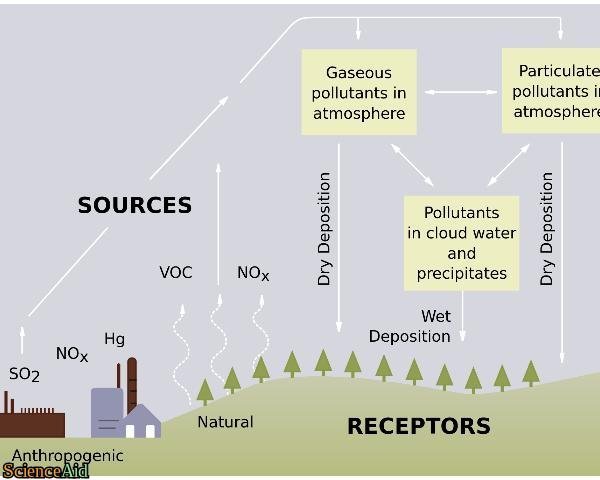
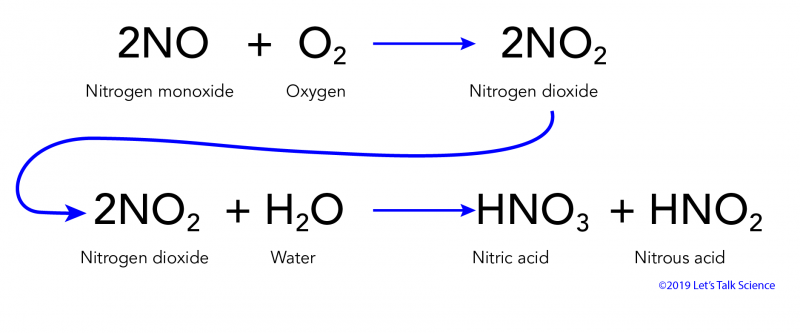
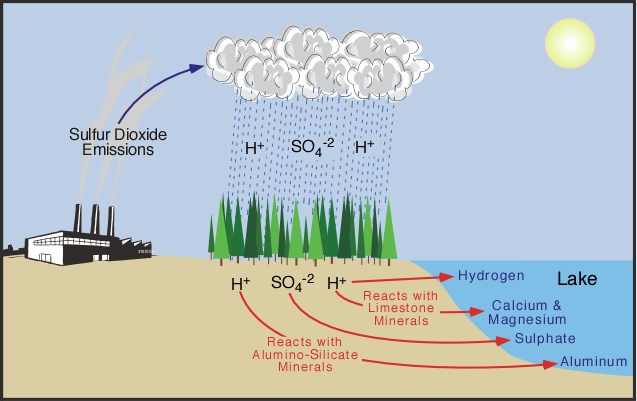
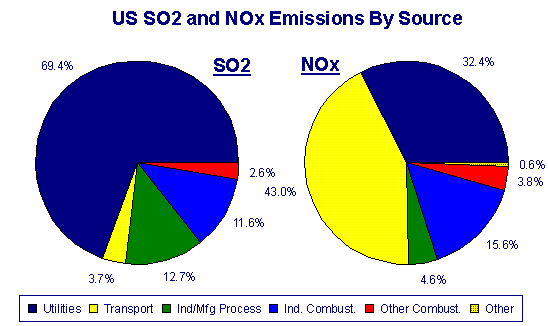
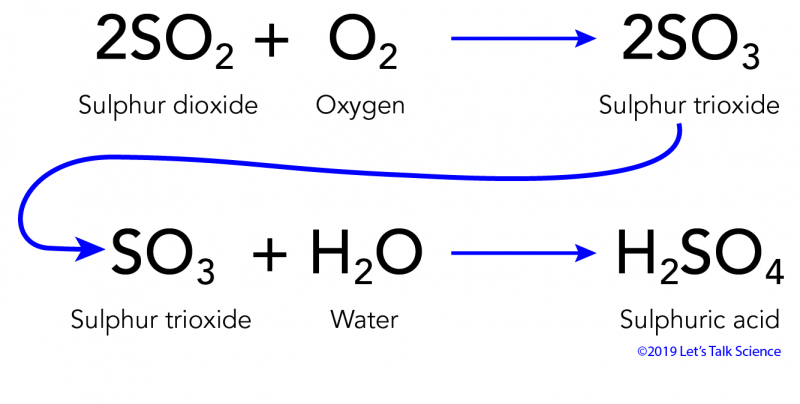
Composition of acid rain. Although sulfur dioxide and carbon dioxide occur in the air natu- rally burning fossil fuels adds more of these chemicals to the air. ACID RAIN Acid rain was discovered in the 19th century by Robert Angus Smith a pharmacist from Manchester England who measured high levels of acidity in rain falling over industrial regions of England and contrasted them to the much lower levels he observed in less polluted areas near the coast. Hydrogen ion H concentration The acidity of rainwater comes from the natural presence of three substances CO2 NO and SO2 found in the troposphere the lowest layer of the atmosphere.
Acidic rain is mainly caused by atmospheric pollutants of sulfur dioxide and nitrogen oxides. Little attention was paid to his. Acid rain or acid deposition is a broad term that includes any form of precipitation with acidic components such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms.
ACID RAIN forms when clean rain comes into contact with pollutants in the air like SULFUR DIOXIDE SO2 CARBON DIOXIDE CO2 and NITROGEN OXIDES NOX. Acid rain or acid deposition is a broad term that includes any form of precipitation that contains acidic components such as sulfuric acid or nitric acid according to the Environmental. The chemical formula of acidic rain is dependent upon the type of acids present.
This can include rain snow fog hail or even dust that is acidic. Acid rain also called acid precipitation or acid deposition precipitation possessing a pH of about 52 or below primarily produced from the emission of sulfur dioxide SO 2 and nitrogen oxides NO x. As acid rain fell it affected everything it touched leaching calcium from soils and robbing plants of.
Beginning in the 1950s acid rain is precipitation in the form of rain snow hail dew or fog that transports sulfur and nitrogen compounds from the high atmosphere to the ground. Normal rain is slightly acidic with a pH of 56 while acid rain generally has a pH between 42 and 44. The two strong acids present in the acid rain are nitrogen oxide and sulphur dioxide.
Acid rain is made up of highly acidic water droplets due to air emissions most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. What Causes Acid Rain. What Causes Acid Rain.