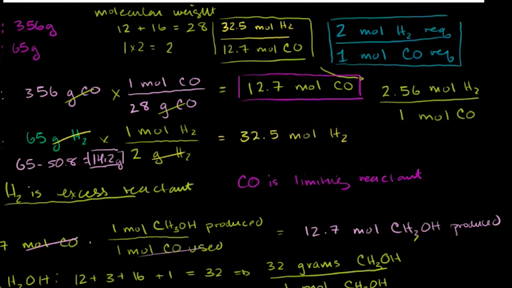Ideal Khan Academy Percent Yield

Limiting reagent Chemical reactions and stoichiometry Chemistry Khan Academy.
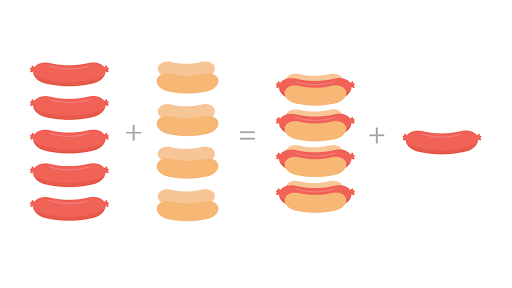
Khan academy percent yield. Gravimetric analysis and precipitation gravimetry. Molecules are left over when one thing runs out. To calculate the mass percent of an element in a compound we divide the mass of the element in 1 mole of the compound by the compounds molar mass and multi.
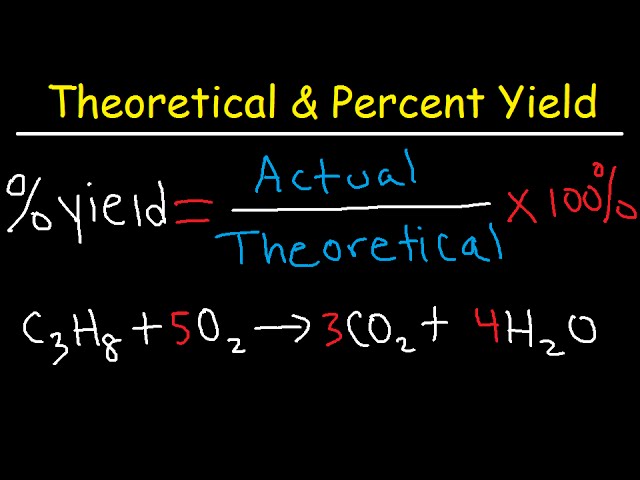
Percent yield is very important in the manufacture of products. The percent yield is the ratio of the actual yield to the theoretical yield expressed as a percentage. 366 write correct formula for compound remembering monatomic charges c.
How To Calculate Percent Yield in a Chemical Equation. When complex chemicals are synthesized by many different reactions one step with a low percent. Carbons molar mass is 12 gmol and oxygens is 16 gmol so the total is 12 16 16 44 Multiply 0834 moles CO 2 x 44 gmol CO 2 367 grams.
What is the percent yield if 0856 g of NH 3 is actually obtained in the lab during the following reaction. Percent Yield Actual Yield and Theoretical Yield. 4NH 3 5O 2 -- 4NO 6H 2 O How many grams of NO are formed if 630g of ammonia react with 180g of oxygen.
Check out more free videos and free sample problems at my free website at wwwchemistr. Problems How To Calculate Theoretical Yield and Percent Yield Limiting Reagent Theoretical Yield and Percent Yield Converting Between Moles Atoms and Molecules Stoichiometry. Is burned and the actual yield of water is 784 g the percent yield in the reaction is.
722 this is the mass percent of magnesium e. This study guide explains how to calculate the mass ratio of sulfuric acid using the following four steps. The percent of nitrogen in magnesium nitride is.