Fine Beautiful Butane Plus Oxygen Balanced Equation

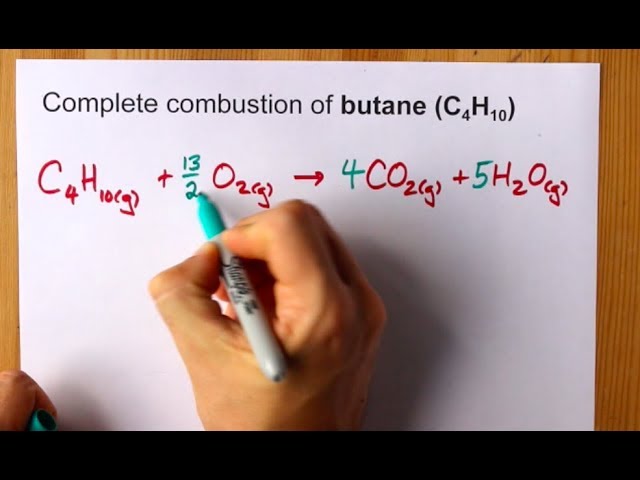
Butane C4H10 reacts with oxygen O2 to form water H2O and carbon dioxide CO2 as shown in the following chemical equation.
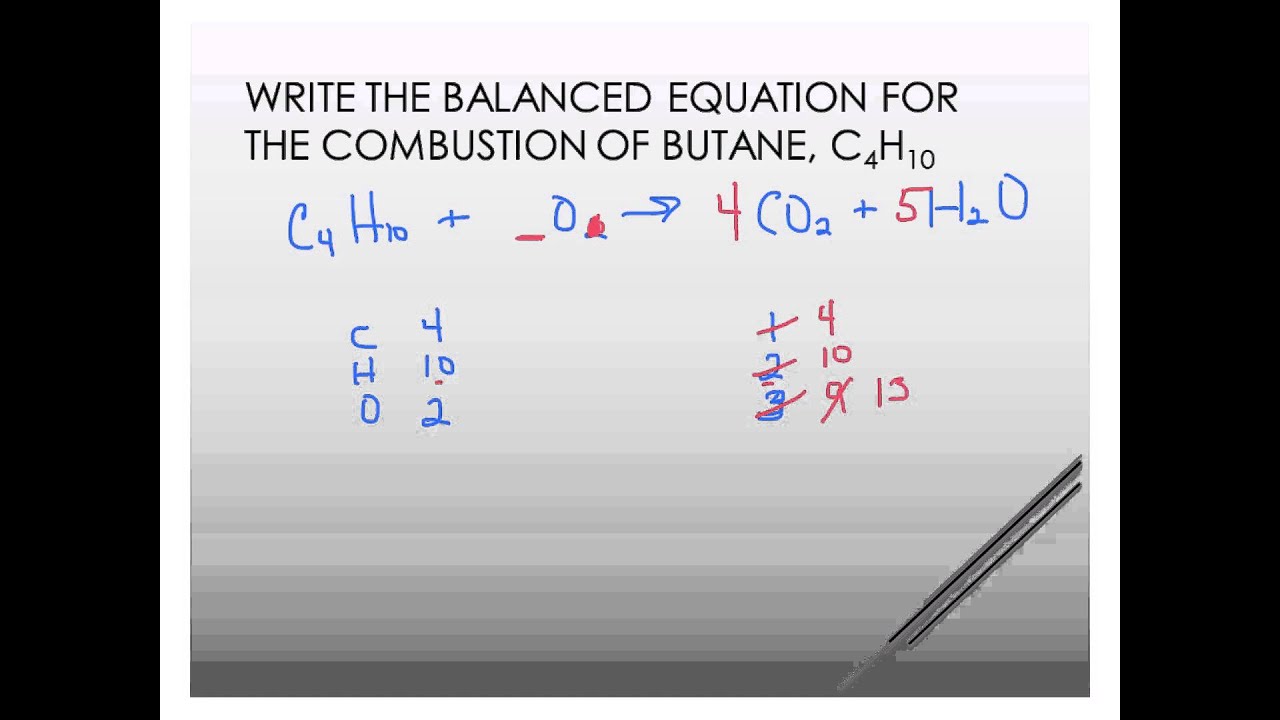
Butane plus oxygen balanced equation. Mol D 50 mol. C4H10 O2 4CO2 5H2O Now the oxygens which appear in one reactant and two products can be balanced. The balanced equation for this reaction is.
The development of processes that operate at conditions where multiple fluid-phase equilibria may occur demands fast and reliable simulation algorithms. In order to balance C4H10 O2 CO2 H2O youll need to watch out for two things. Complete combustion does NOT give carbon monoxide or sootCheck me out.
Gaseous butane C 4 H 10 reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. Propane C3H8 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O. From the above balanced chemical equation of combustion of butane moles of oxygen are required to burn 1 mol of butane.
Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. C4H10 132O2 4CO2 5H2O This equation is valid on a molar level One mole butane reacts with six-and-a-half mols of oxygen gas to give four mols carbon dioxide and five mols water. Now the oxygens which appear in one reactant and two products can be balanced.
Given the balanced equation for the reaction of butane and oxygen. 2C4H10 g 13O2 g rightarrow 10H2O g 8CO2 g Calculate the. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water.
The success of these algorithms depends on the correct prediction of the number and compositions of the phases present at a given temperature pressure and overall fluid composition. First be sure to count all of C H and O atoms on each side of the. Ionic charges are not yet supported and will be ignored.