Ideal Evidence Of Chemical Change In An Iron Nail Rusts

The following reaction occurs.
Evidence of chemical change in an iron nail rusts. -grass in a yard is cut. The vinegar removes the irons protective coating causing the metal to rust. For Example when the iron is exposed to air and moisture rust formation takes place.
The formation of a reddish brown flakes which loosely adheres to the iron is called rust. When iron reacts with vinegar the metal rusts and causes an exothermic chemical reaction which produces heat. This must be a chemical change.
Lithium in cell phone. The exposure of iron or an alloy of iron to oxygen in the presence of moisture leads to the formation of rust. Evidence of Chemical Change 2.
E Iron and sulfur form a shiny nonmagnetic grey substance on heating. This is due to the formation of iron oxide which is the reaction between iron and oxygen. Changing from a gas to a liquid is a PHYSICAL CHANGE.
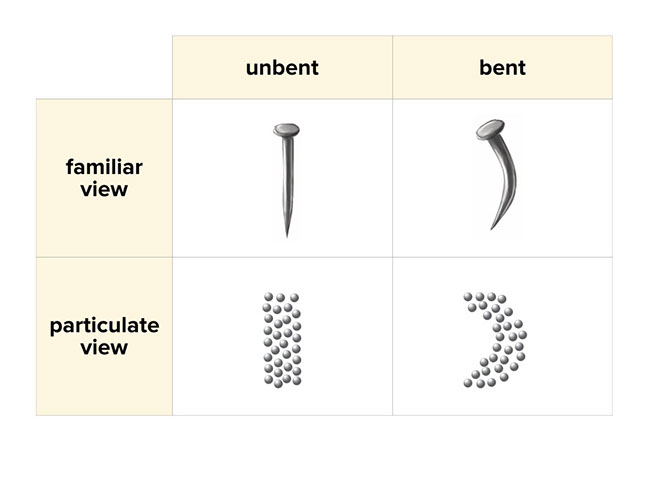
The evidence of chemical reaction in the rusting of an iron nail is a color change. However the rate of a reaction can be changed. The dark grey nail changes color to form an orange flaky substance the rust.
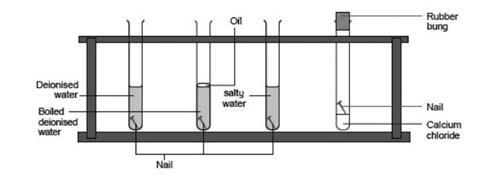
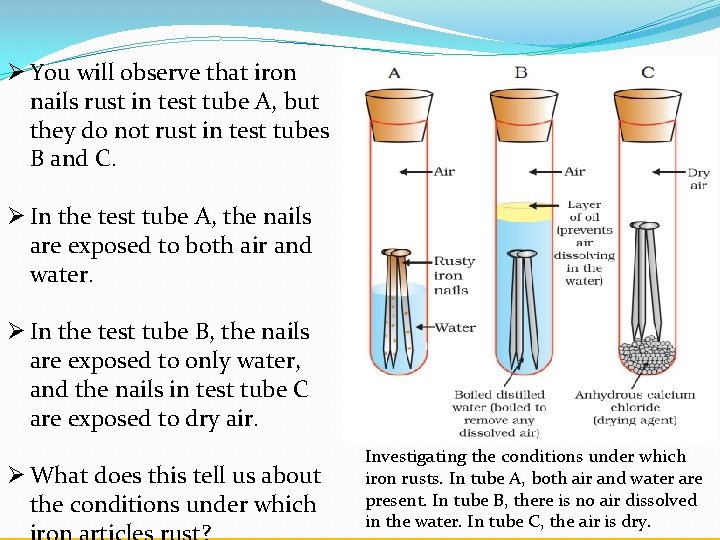
D A piece of paper burns. An iron nail rusts. Water mixes with carbon dioxide in the air forming a carbonic acid that dissolves the iron.