Exemplary Examples Of Displacement Reactions

Here both iron and copper have the same valence.
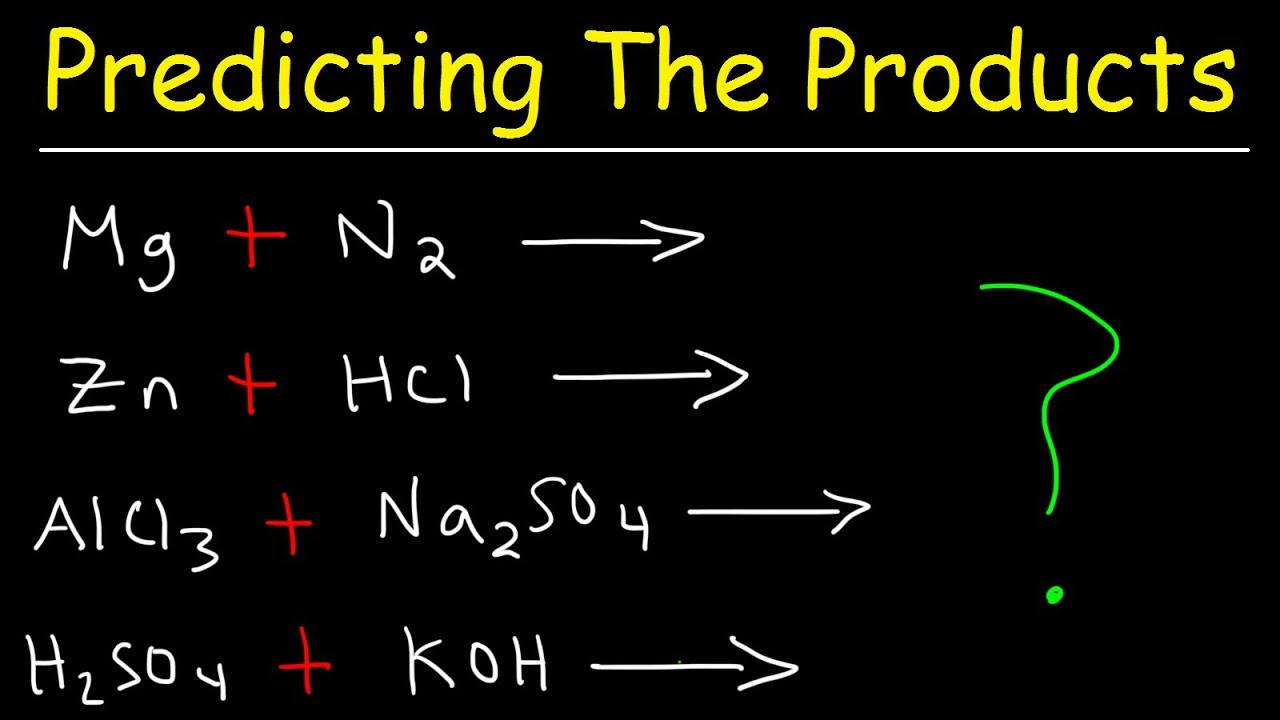
Examples of displacement reactions. A displacement reaction is the one wherein the atom or a set of atoms is displaced by another atom in a molecule. Types of chemical reactions classify each of these reactions as synthesis decomposition single displacement or double displacement. And are halogens and is a cation.
The above equation exists when A is more reactive than B. Zinc displacing iron ions from iron II sulfate solution. Maybe you would like to learn more about one of these.
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. A B-C A-C B. Some other examples of displacement reactions which can occur are.
When pieces of iron kept in the moisture then the moisture in the form of rust covers these pieces of iron and slowly displace the iron. A double displacement reaction is a type of reaction in which two reactants exchange ions to form two new compounds. Chemical Reactions and Equations Class 10 Part 6 CBSE Class 10 Chemistry 100 FREE Notes More Examples of Displacement ReactionTable of Reactivity.
Zinc displacing iron ions from iron II sulfate solution nickel displacing copper from copper II nitrate solution copper. Some other examples of displacement reactions which can occur are. In a displacement reaction.
A displacement reaction is the one wherein the atom or a set of atoms is displaced by another atom in a molecule. Check spelling or type a new query. An example is the reaction between iron and copper sulfate to produce iron sulfate and copper.