Wonderful Conditions Required For Rusting Of Iron

Aluminium on the other hand does not corrode easily because its surface is protected by a layer of aluminium oxide.
Conditions required for rusting of iron. The corrosion of iron. Answer verified by Toppr Upvote 52. Students may also use galvanised nails in all or some of the conditions and also may use the magnesium and copper strips to.
Conditions for Rusting Iron and steel rust when they come into contact with water and oxygen. I What causes rusting of iron Design an activity to show the conditions needed from CHE INORGANIC at University of Madras Institute of Distance Education. Both water and oxygen are needed.
Factors that Affect the Rusting of Iron. The usually accepted view has been that iron will not rust unless carbonic acid is present. The essential requirements for rusting to occur under standard conditions are Iron steel steel is alloyed iron used in engineering.
For example to avoid corrosion iron and steel tools and machine parts are rubbed with grease or oil. Essentially just combining iron with oxygen wont do much if the air is dry. This causes the pipes to carry brown or black water containing an unsafe amount of iron oxides.
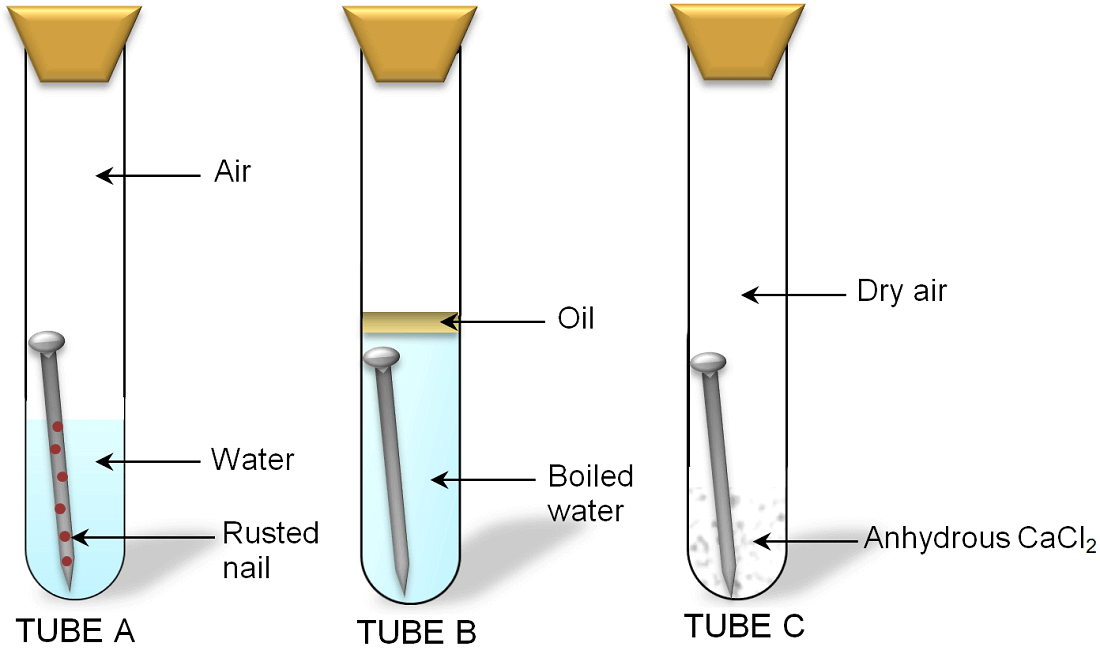
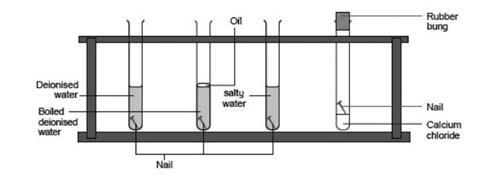
Each boiling tube holds a nail in water salty water water with oil on top or just oil. Given sufficient time any iron mass in the presence of water and. This shows that for rusting of iron both air and water are necessary.
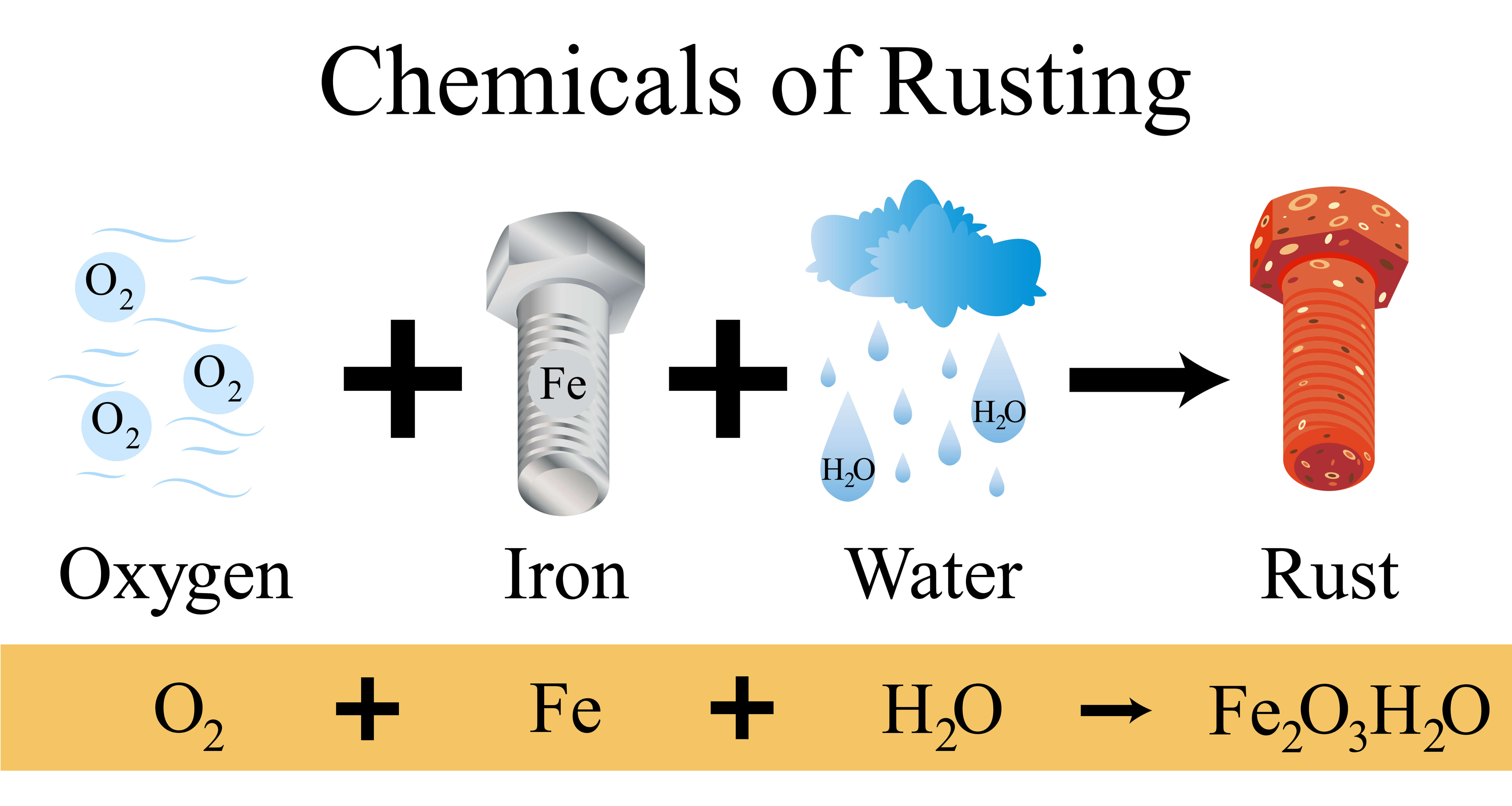
Rust is an iron oxide a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moistureRust consists of hydrous ironIII oxides Fe 2 O 3 nH 2 O and ironIII oxide-hydroxide Fe 2 O 3 OH FeOH 3 and is typically associated with the corrosion of refined iron. Water is usually present in the form of water vapour and oxygen is always present in the normal atmosphere. Half fill two test tubes with water.