Ace Meaning Of Displacement Reaction
It can be represented generically as.
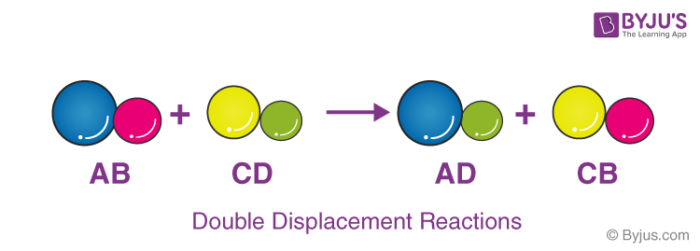
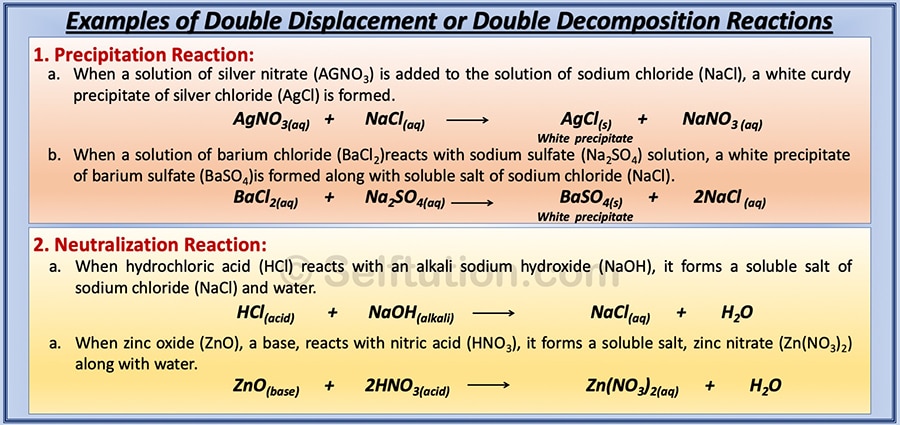
Meaning of displacement reaction. What is double displacement reaction definition. Better learning for better results. Double Displacement Reaction A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
Displacement reaction does not occur because copper metal is less reactive than iron metalSo a less reactive metal copper cannot displace a more reactive metal iron from its salt solutioniron sulphate solution. The chemical bonds between the reactants may be either covalent or ionic. Displacement reactions involve a metal and a compound of a different metal.
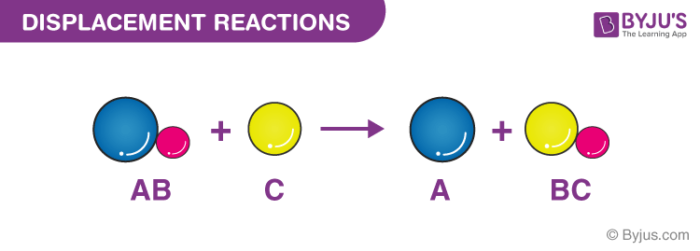
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. And what they in solutions. Displacement comes about when a person directs or displaces their negative emotions impulses frustrations and reactions onto a less threatening subject in order to avoid negative consequences.
Usually a double displacement reaction results in precipitate formation. Learn the basics about Displacement reactions and reactions in solutions. Here A is a more reactive element and B is less reactive element forming a compound with C BC.
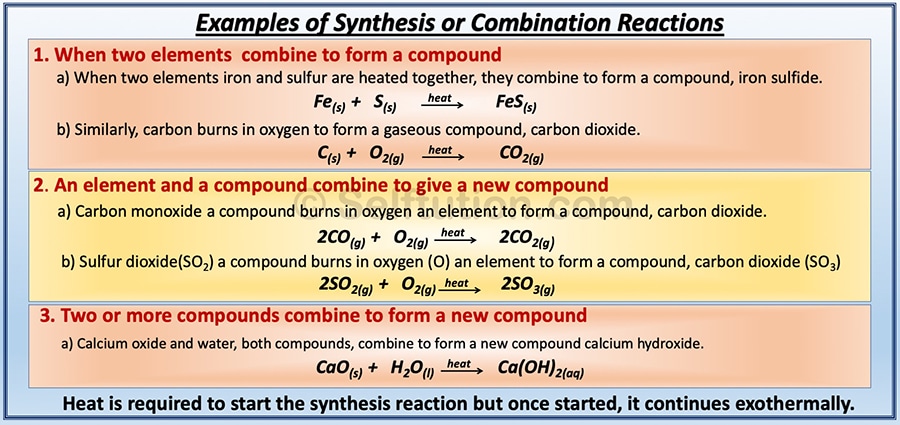
Displacement reaction synonyms displacement reaction pronunciation displacement reaction translation English dictionary definition of displacement reaction. There are two types of displacement reactions. The chemical bonds between the reactants may be either covalent or ionic.
Usually a double displacement reaction results in precipitate formation. A displacement reaction is a type of reaction in which part of one reactant is replaced by another reactant. And the general equation of these reactions looks something like this.







/attachments/Displacement%20Reactions%20of%20Metals%20(1)%20-%20Model%201.gif)
