Smart Methanol Combustion Reaction Balanced

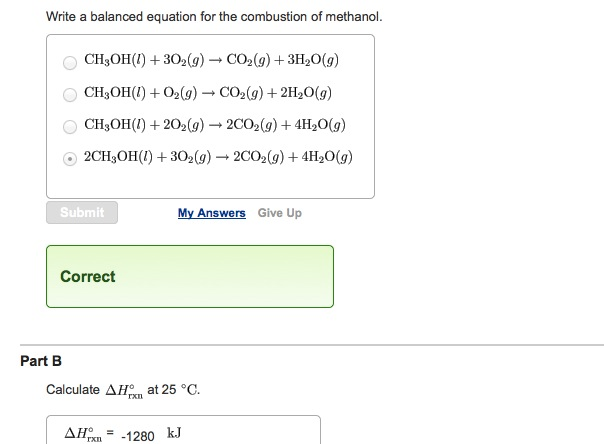
Write a balanced equation for the combustion of methanol.
Methanol combustion reaction balanced. The reaction is the combustion of methanol CH3OH. 2 CH3OH 3 O2 2 CO2 4 H2O. 2CH3OHl 3O2g 2CO2g 4H2Ol.
Methanol is occasionally used to fuel internal combustion engines. Chemistry QA Library According to the following combustion reaction pure methanol liquid undergoes complete combustion with excess oxygen. Write a balanced equation for the reaction using the AH notation - 2 marks b.
Express your answer as a chemical. Write a balanced equation for the complete combustion of liquid methanol CH3OH. 2 CH3OH 3 O2 2 CO2 4 H2O.
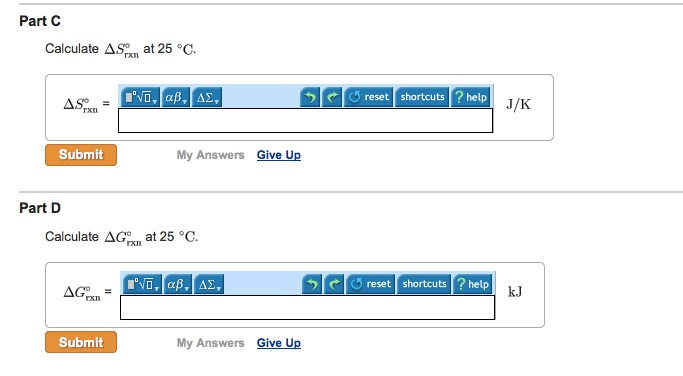
It burns forming carbon dioxide and water. CH3OHl 3O2g rightarrow CO2g 3H2Og CH3OHl O2g rightarrow CO2g 2H20g CH3OHl 2O2g rightarrow 2CO2g 4H20g 2CH3OHl 3O2g rightarrow 2CO2g 4H20g Correct Calculate Delta H degree rxn at 25 degree C. Hydrogen and methanol have both been proposed as altern atives to hydrocarbon fuels.
AA-grade methanol is produced at a reaction pressure of 70 bar a reaction temperature of 250 C a molar H 2 CO 2 ratio of 30 and application of commercial CuZnOAl 2 O 3 catalyst. 1 The complete combustion of methanol. Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels.
Methanol oxygen carbon dioxide water 2CH 3 OH l 3O 2g 2CO 2g 4H 2 O l. Since only water is present in a nonstandard state the enthalpy for the reaction is equal to the enthalpy of formation of water. If 3820 L of pure carbon dioxide was recovered removed at STP how many ML of methanol must have reacted.