First Class Incomplete Combustion Balanced Equation

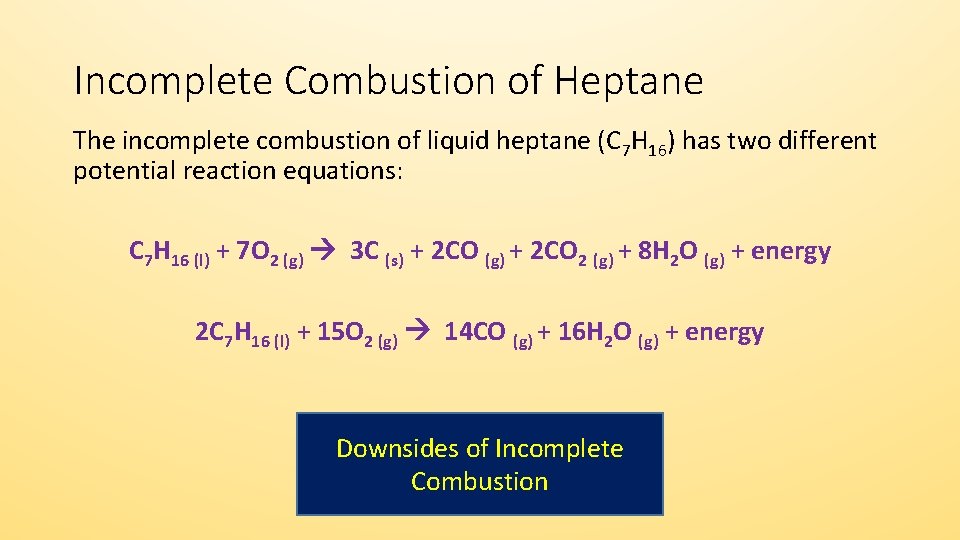
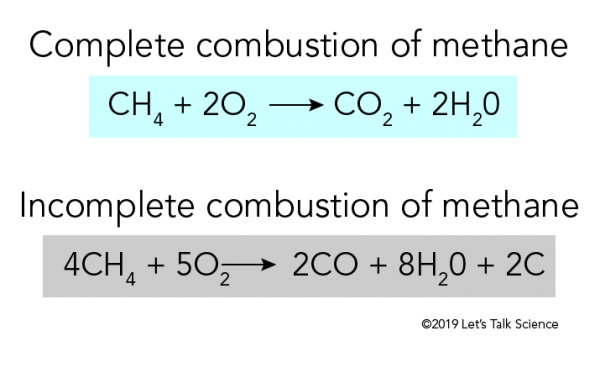
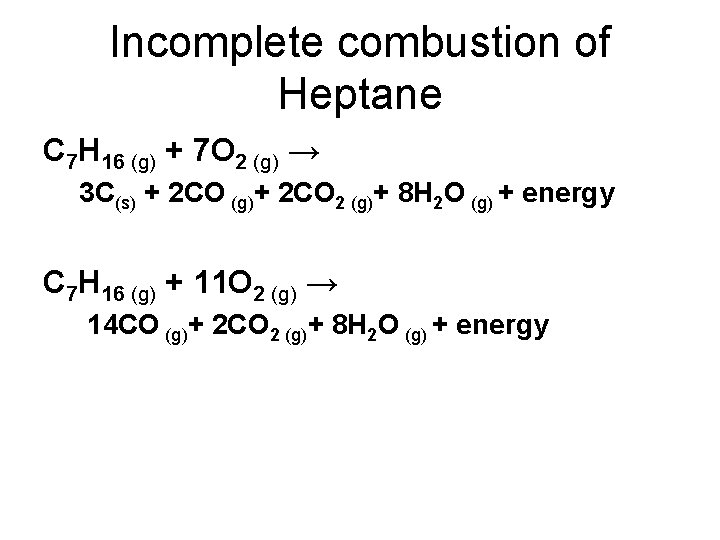
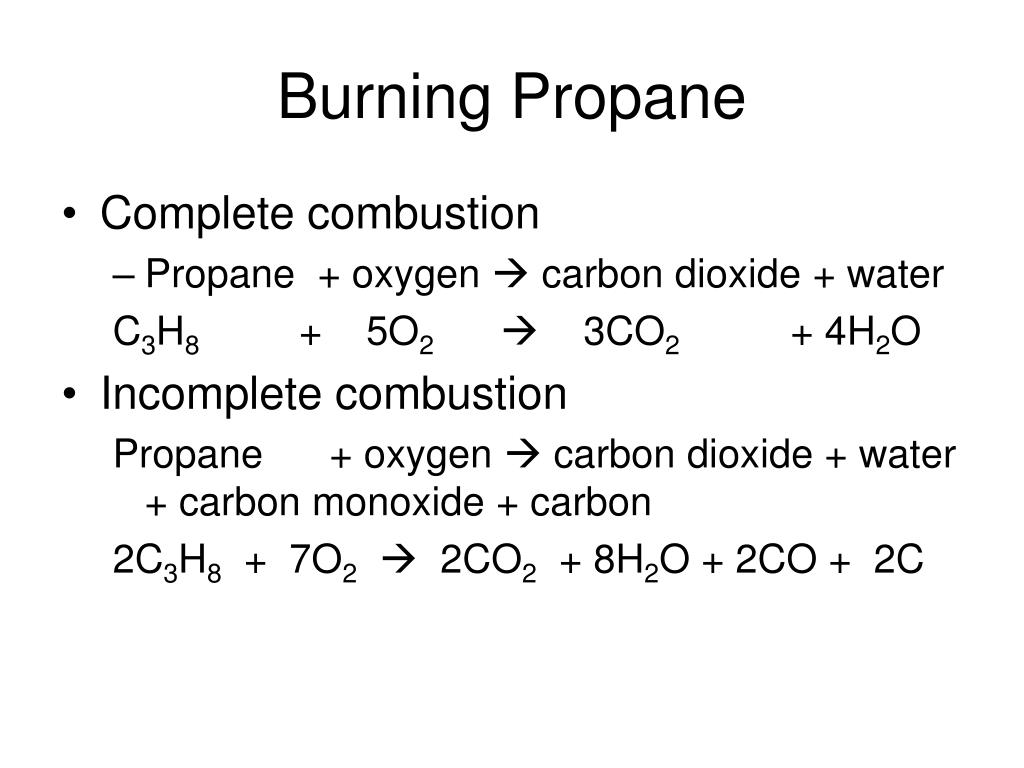
Incomplete combustion means burning in a lack of air not enough oxygenIf there is not enough oxygen available for all the carbon to turn into carbon dioxide see complete combustion then some or all of the carbon turns to carbon monoxideThis happens with.
Incomplete combustion balanced equation. Draw the products of the given reactions yout What is the name of the product above. Incomplete combustion of pentene. The chemical formula of ethanol is C2H5OH By complete combustion of ethanol carbon dioxide and water vapour are produced.
As we know that the hydrocarbons can undergo complete or incomplete combustion that depends on the amount of oxygen available. Do not use half-numbers as coefficients. Chemistry questions and answers.
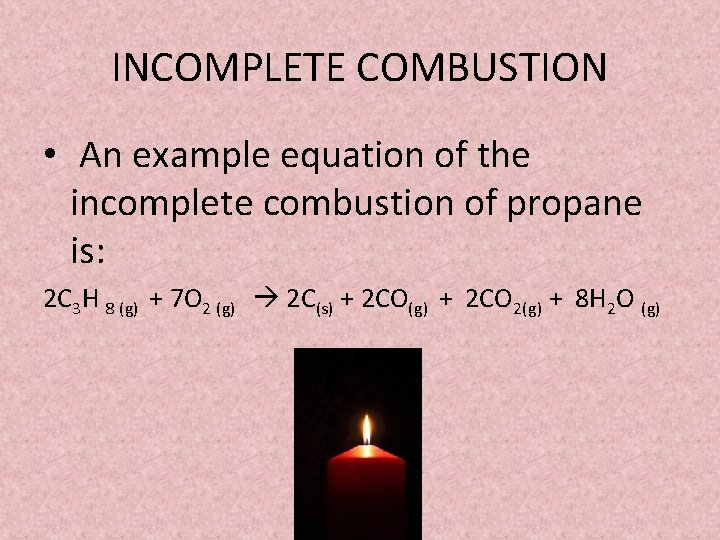
Complete combustion of pentene. C2H5OH 3O2 2CO2 3 H2O complete combustion. INCOMPLETE COMBUSTION An example equation of the incomplete combustion of propane is.
The equation for incomplete combustion of propane is. The Incomplete Combustion of Natural Gas - Methane. It takes place when there is a good supply of oxygen.
Chem 1205 Question 7-8. Before we balance the incomplete combustion of pentane lets remember first that the balanced equation for the complete combustion for pentane will be written as follows. Could it happen the reaction should be described by the equation C2H6 2 O2 CO C 3 H2O From a purely stoichiometric standpoint it is to be pointed out that in the absence of the arbitrary constraint concerning the equal amounts of C.
But by incomplete combustion of ethanol carbon monoxide and water vapour are produced. Recognizing the characteristics and balancing incomplete combustion reactions. For example using ethane C2H6.