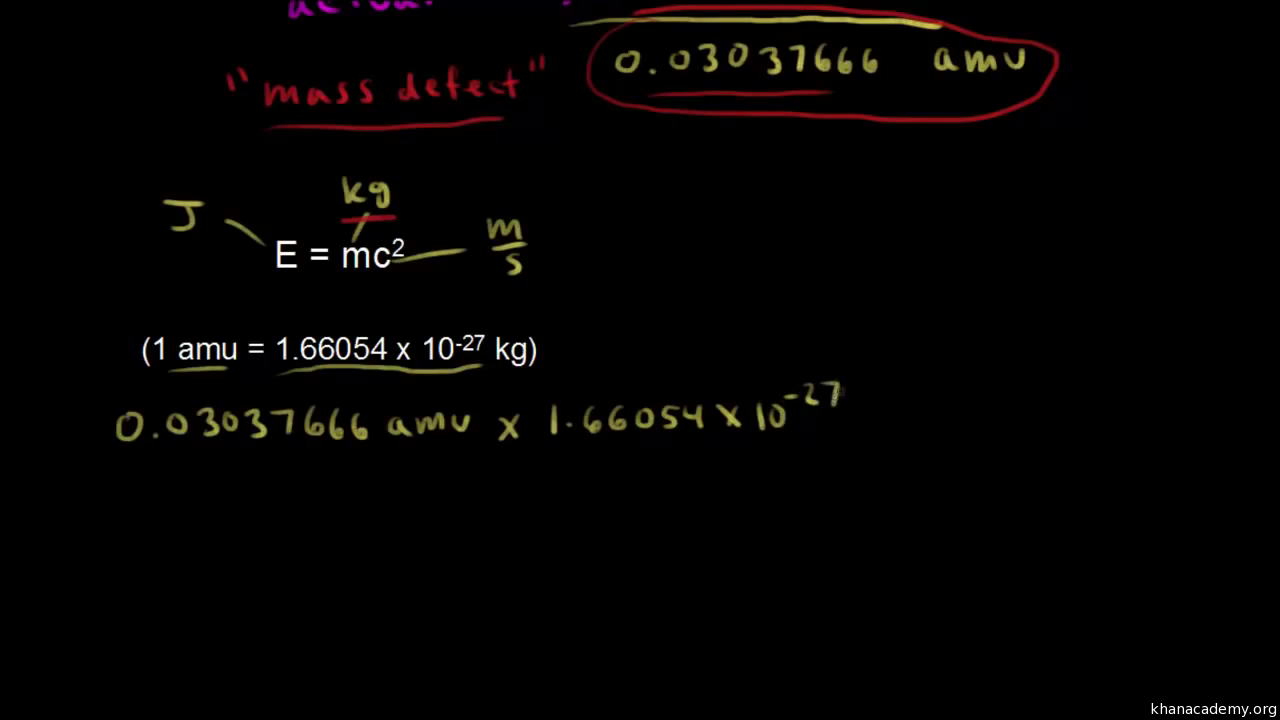Fabulous Formula Of Mass Defect

Does an electron have any significant mass.
Formula of mass defect. Therefore the total mass 201456 201743 403190 amu. The actual mass of the nucleus the composition of. Mass defect is expressed in unit amu or Kg.
The mass defect 𝚫M can be calculated by subtracting the original atomic mass M A from the sum of the mass of protons m p 100728 amu and neutrons m n 100867 amu present in the nucleus. Discovered by Albert Einstein in 1905 it can be explained using his formula E mc2 which describes the equivalence of energy and mass. 1 amu is equal to 166 x 10 -27 Kg.
Binding Energy Formula Binding Energy mass defect x c2 where c speed of light in vacuum c 29979 x 10 8 ms. The binding energy of a system can appear as extra mass which accounts for this difference. The mass defect and binding energy are related by Albert Einsteins formula E mc 2.
This mass defect converted or transformed into energy by the Einstein relativity equation in. The mass of an atomic nucleus is usually less than the sum of the individual masses of the constituent protons and neutrons and this difference of mass is acknowledged as the mass defect and signifies the energy that is to be released if a nucleus form. In 1905 Einstein developed the special theory of relativity.
Hence 403190 400150 003040 amu is the mass defect of helium. One of the implications of this theory was that matter and energy are interchangeable with one another. The difference between the mass of a nucleus and the sum of the masses of the nucleons of which it is composed is called the mass defect.
Subtract the actual mass of the nucleus from the combined mass of the components to obtain the mass defect. The Mass Defect formula is defined as the difference between the actual atomic mass and the predicted mass and is represented as m Zm9 A-Zmn-matom or mass_defect Atomic numberMass of proton Mass number-Atomic numberMass of neutron-Mass of atom. The mass defect is given by the formula Here mn mp -represent combined mass of proton and neutron and mo - denotes original mass of atom The nuclear binding energy accounts for the noticeable difference between the actual mass of atoms nucleus and expected massThe is the main energy which helps to hold the nucleus together.