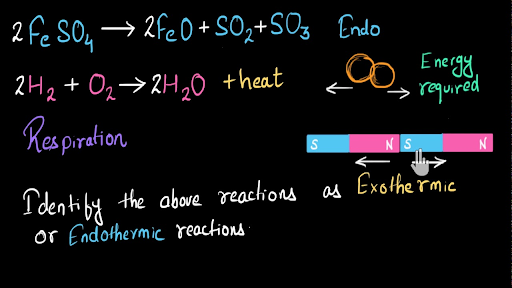Stunning Exothermic Reaction General Formula

It is the mixture of iron oxide powder aluminum powder and starts reacting when it is heated.
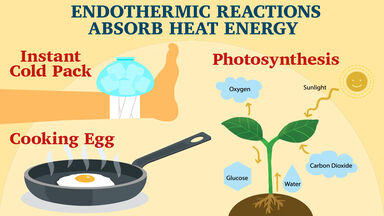
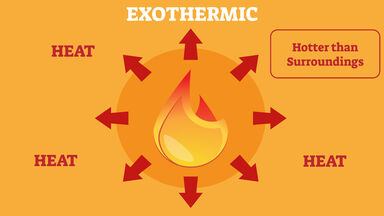
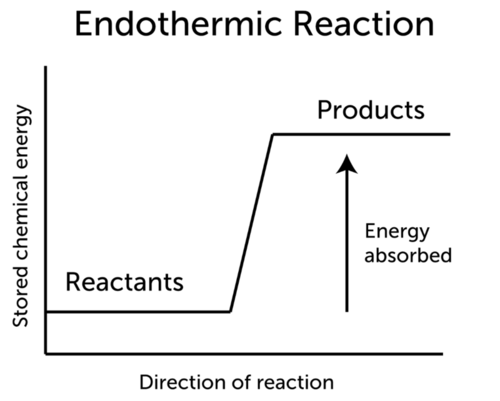
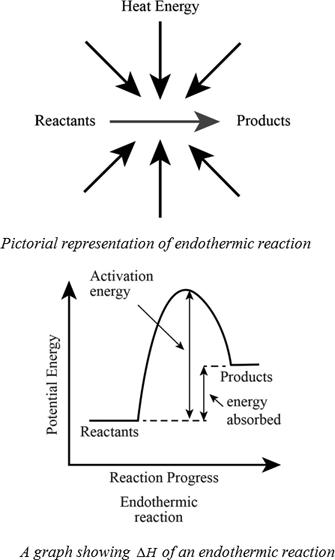
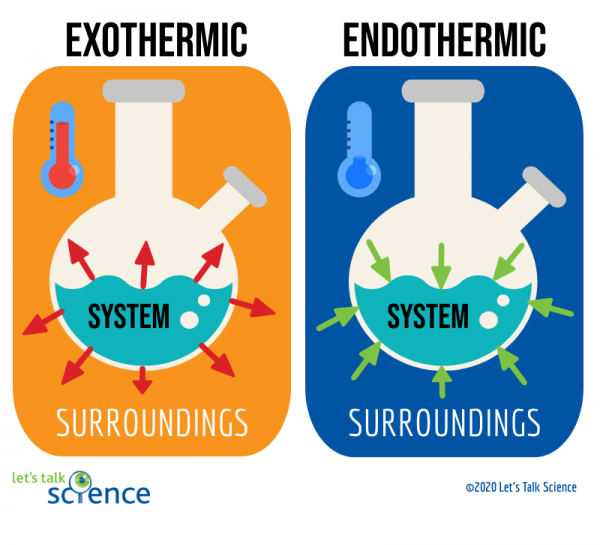
Exothermic reaction general formula. For example I can bet that H 2- 2H is endothermic because I know that the H 2 has lower energy than the 2 Hs. The word exothermic literally means turning out heat Energy often in the form of heat is released as an exothermic reaction occurs. The exothermic reaction is the opposite of an endothermic reaction.
Reactants products energy. The general equation for an exothermic reaction is. Reactants Products Energy What is an Exothermic Reaction.
Reactants Products Energy. Assume a coolant temperature at the. Exothermic Reactions In an exothermic reaction it takes less energy to break bonds in the reactants than is released when new bonds form in the products.
As a result the. Step 5- Since ΔH is negative 23 kcal the reaction is exothermic. Consider an exothermic reaction where the coolant stream enters at the end of the reactor at a temperature Ta0 say 300 K.
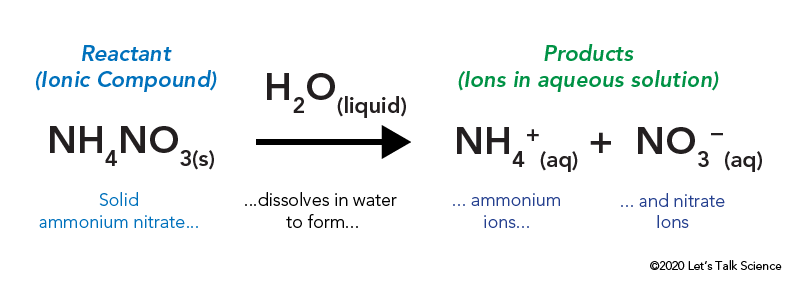
Step 4- Set up the table see below and apply the formula for enthalpy change. This reaction is an exothermic reaction and the equation is given below. It is the opposite of an endothermic reaction.
Step 1- First look at the equation and identify which bonds exist on in the reactants bonds broken. It releases energy by light or heat to its surrounding. Reactants products energy An exothermic reaction is a chemical or physical reaction that releases heat.