Nice Energy Balance Equation For Open System

As well as heat and work.
Energy balance equation for open system. D N i δ N i l e a k δ N i r e a c t i o n Also energy transfers are always path dependent since they are not a systems property but depend on the process path. Closed and Open Systems. In this example we will calculate the global average temperature without greenhouse gases and show the effect which greenhouse gases have on the earths energy balance.
It is assumed that the rate at which heat is exchanged between the two streams is much larger than possible heat exchange between any or both of the streams and the environment and this exchange with the environment is therefore ignored. Heat is added to a constant pressure system and a constant vo. Similar to mass balances studied previously a balance on energy is crucial to solving many problems.
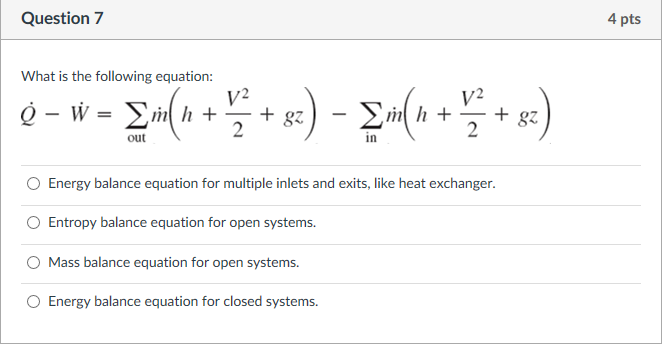
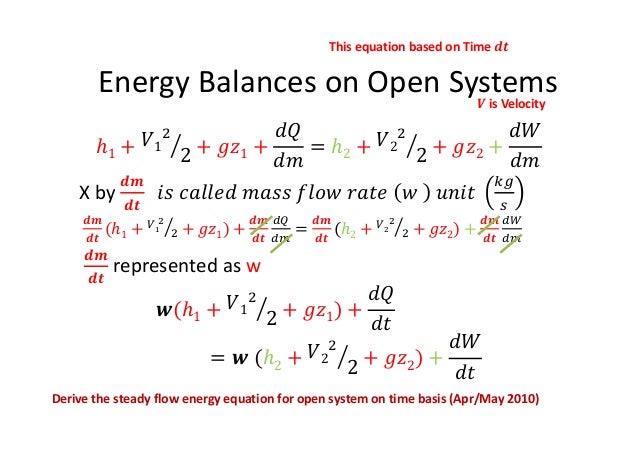
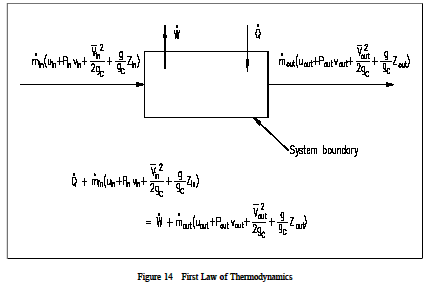
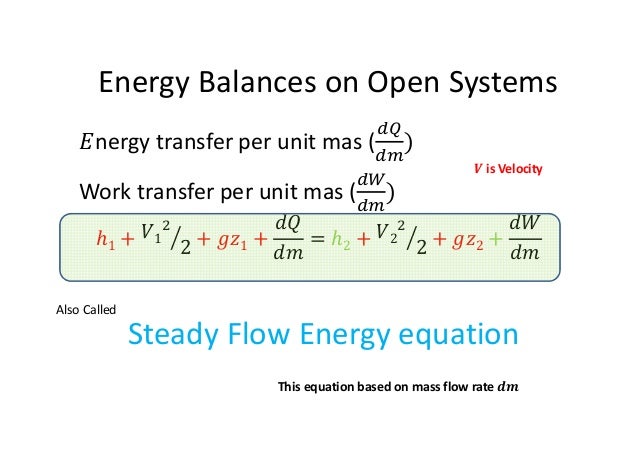
The big nasty energy balance equation at the bottom is the one we are most interested in right now. In formulating the energy balance equation for the open system we must consider energy transfer involved with materials in transit. In an open system work must be done to push input fluid streams at a pressure P in and flow rate into the system.
The First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. This is one to commit to memory. Your feedback is important to me in.
Edge - Edw H - internal energy dKE - Kinetic energy PE - Potential energy E do total heat transfer s dw total amount of works for windmill d HJ - 0 because internal energy of air will be chang megligibally. 001Rate Form to SS. Here is a quick review of mass and energy balances for open and closed systems.
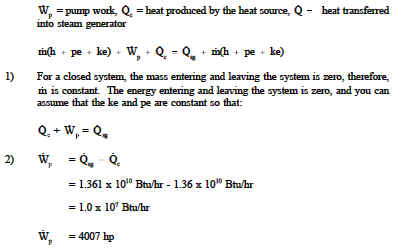
The First Law for the Closed System. Equation g is an energy balance over the system alone. In this video I derive the overall energy balance for an open and closed system involving shaft work work done by fluid flow and work done by expansion an.